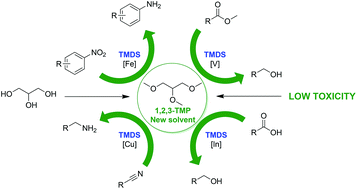
1,2,3-Trimethoxypropane (1,2,3-TMP) was prepared from glycerol in one step in good yield and selectivity by phase transfer catalysis. According to OECD guidelines, a toxicity study was realized for this compound. It revealed that 1,2,3-TMP has a low acute toxicity, no skin sensitization, no mutagenicity and no ecotoxicity in an aquatic environment. This compound was also used as a solvent for the reduction of organic functions using either aluminium hydride or 1,1,3,3-tetramethyldisiloxane (TMDS) as a benign hydride source. In particular, a new process for the reduction of nitriles to amines in 2-MeTHF and in 1,2,3-TMP was developed, using TMDS in combination with copper triflate (Cu(OTf)2).

 Please wait while we load your content...
Please wait while we load your content...