 Open Access Article
Open Access ArticleHass avocado modulates postprandial vascular reactivity and postprandial inflammatory responses to a hamburger meal in healthy volunteers
Zhaoping Li*, Angela Wong, Susanne M. Henning, Yanjun Zhang, Alexis Jones, Alona Zerlin, Gail Thames, Susan Bowerman, Chi-Hong Tseng and David Heber
UCLA Center for Human Nutrition, David Geffen School of Medicine at UCLA, 900 Veteran Avenue, Room 12-217 12-217 Warren Hall Box 951742, Los Angeles, California 90095, USA. E-mail: zli@mednet.ucla.edu; Fax: +1 310 206-5264; Tel: +1 310 206-1987
First published on 19th November 2012
Abstract
Hass avocados are rich in monounsaturated fatty acids (oleic acid) and antioxidants (carotenoids, tocopherols, polyphenols) and are often eaten as a slice in a sandwich containing hamburger or other meats. Hamburger meat forms lipid peroxides during cooking. After ingestion, the stomach functions as a bioreactor generating additional lipid peroxides and this process can be inhibited when antioxidants are ingested together with the meat. The present pilot study was conducted to investigate the postprandial effect of the addition of 68 g of avocado to a hamburger on vasodilation and inflammation. Eleven healthy subjects on two separate occasions consumed either a 250 g hamburger patty alone (ca. 436 cal and 25 g fat) or together with 68 grams of avocado flesh (an additional 114 cal and 11 g of fat for a total of 550 cal and 36 g fat), a common culinary combination, to assess effects on vascular health. Using the standard peripheral arterial tonometry (PAT) method to calculate the PAT index, we observed significant vasoconstriction 2 hours following hamburger ingestion (2.19 ± 0.36 vs. 1.56 ± 0.21, p = 0.0007), which did not occur when the avocado flesh was ingested together with the burger (2.17 ± 0.57 vs. 2.08 ± 0.51, NS p = 0.68). Peripheral blood mononuclear cells were isolated from postprandial blood samples and the Ikappa-B alpha (IκBα) protein concentration was determined to assess effects on inflammation. At 3 hours, there was a significant preservation of IκBα (131% vs. 58%, p = 0.03) when avocado was consumed with the meat compared to meat alone, consistent with reduced activation of the NF-kappa B (NFκB) inflammatory pathway. IL-6 increased significantly at 4 hours in postprandial serum after consumption of the hamburger, but no change was observed when avocado was added. Postprandial serum triglyceride concentration increased, but did not further increase when avocado was ingested with the burger compared to burger alone despite the added fat and calories from the avocado. These observations are suggestive of beneficial anti-inflammatory and vascular health effects of ingesting added Hass avocado with a hamburger patty.
Introduction
Atherosclerosis is one of the main causes of morbidity and mortality in the Western world. Several hypotheses have been articulated to explain the initiating events in atherosclerosis, but its pathogenesis seems to be multifactorial.1 Over the past twenty years, the contribution of oxidation of lipoproteins including cholesterol and LDL cholesterol to the development of atherosclerosis has been demonstrated in both basic and clinical studies.2 Endothelial dysfunction contributes to atherosclerosis3 and is characterized by a decreased bioavailability of nitric oxide and increased expression of proinflammatory cytokines.4 The use of antioxidants and anti-inflammatory natural products from dietary sources, such as fruits and vegetables including avocados, has been proposed as an adjunct to other preventive measures such as achieving and maintaining a healthy body weight, and controlling blood cholesterol levels with drugs.High-calorie meals rich in saturated fat and carbohydrate can lead to transient exaggerated elevations in blood glucose, free fatty acids, and triglycerides. This condition, termed postprandial dysmetabolism, generates excess free radicals (or reactive oxygen species). The ensuing oxidative stress triggers a biochemical cascade throughout the circulation, inducing inflammation, and endothelial dysfunction. These postprandial changes, when repeated multiple times each day, can create a milieu conducive to the development of atherosclerotic risk factors and coronary heart disease (CHD), the main cause of morbidity and mortality in the Western world.5–8
The stomach is a prime location for interactions of these antioxidant compounds with lipids and other food constituents prone to oxidation. The stomach acts as a bioreactor and gastric fluid as a medium for further dietary lipid peroxidation and/or antioxidation.9 The gastrointestinal tract is constantly exposed to dietary oxidized food compounds produced during the processing and storage of foods5,10 or during their digestion in the stomach.9 The idea that the gastrointestinal tract is the location for the protective activity of antioxidants was presented by Halliwell et al.11
The avocado contains monounsaturated fats, lutein, glutathione, vitamin E, and phenolic antioxidants.12,13 It has a similar calorie density to other fruits and vegetables due to its high water content. Avocados are rich in oleic acid, similar to what is found in olives and olive oil. However, there is limited information on the anti-oxidant/anti-inflammatory properties of avocados in humans. It has been reported that avocado-enriched diets resulted in improvements in lipid profiles when compared to a high-carbohydrate diet in healthy subjects14 and those with Type 2 DM.15 In addition it was demonstrated that total cholesterol and LDL cholesterol concentrations were significantly decreased in mildly hypercholesterolemic patients consuming an avocado-rich diet.16
In the present pilot study we examined the effect of avocado added to hamburger meat, a common culinary combination, on postprandial vascular health and anti-inflammatory activity in healthy subjects.
Materials and methods
Study subjects
The study utilized a randomized crossover design and included 11 healthy male volunteers. The study protocol was approved by the University of California Los Angeles (UCLA) Institutional Review Board. All subjects gave written informed consent prior to any study procedures being conducted. Subjects were excluded if they had metabolic disorders, were taking dietary supplements, smoked >1 cigarette per day, exercised heavily (aerobic exercise more than four times for 30 min per week), or drank more than 2 glasses of wine, 2 cocktails or two beers per day. Each subject was seen at the research clinic on two separate occasions separated by at least one week. Subjects consumed, in a random order, two different test meals consisting of either: (a) a ground beef patty seasoned with salt, or (b) a ground beef patty seasoned with salt and 68 grams of avocado. The subjects were asked to avoid eating meat, poultry, fish or avocado products for 3 days before the day of each of the two experimental visits.Test meals
Hamburger patties were prepared in the UCLA General Clinical Research Center kitchen. The beef with 10% of fat was weighed, minced in a large Kitchen Aid mixer bowl for 2 minutes at the lowest setting mixed with 1 g of salt. Using a 5 ¾ inch ring mold the meat was divided into 250 g patties and flattened. They were cooked to an internal temperature of 77 degrees centigrade, frozen and packaged in the UCLA General Clinical Research Center kitchen. They were delivered frozen to the UCLA Center for Human Nutrition. The patties were then reheated in their packaging using a boiler at the UCLA Center for Human Nutrition to approximately 60°C. The two test meals consisted of either a burger alone or same burger with avocado with a glass of water (200 ml). Fresh, ripe Hass avocados (provided by the Hass Avocado Board) were sliced into halves; 68 grams were scooped from one half and weighed. The avocado portion was prepared into a mash and added to the hamburger.Study protocol
Participants arrived at the UCLA Center for Human Nutrition after fasting for 10 hours. After an indwelling catheter was inserted into the vein of the forearm and a blood sample was taken, the first measurement of peripheral arterial tonometry (PAT) was performed. The subjects were then provided either the burger or burger and avocado to be eaten within 30 min. Blood samples were drawn again at 1, 2, 3, 4, 5, 6 h after the meal for triglyceride and nitric oxide analyses. PAT was repeated after 2 hours. Urine was collected in a 2 L container for 6 h after the meal. Urine volume was recorded and aliquots were taken for analysis of nitric oxide and creatinine.Peripheral arterial tonometry
Using established methods,17 a blood pressure cuff was placed on one upper arm (study arm), while the other arm served as a control (control arm). Peripheral arterial tonometry probes were placed on one finger of each hand for continuous recording of the PAT signal. After a 10 min equilibration period, the blood pressure cuff will be inflated to suprasystolic pressures for 5 min. Then the cuff was deflated, while PAT recording was continued for 10 min. Reactive hyperemia-peripheral arterial tonometry (RH-PAT) data were analyzed by a computer in an operator-independent manner as previously described.18Biochemical analysis
EDTA plasma and serum were separated from whole blood by centrifugation (910 g, 15 min), frozen, and kept at −80°C. Serum triglyceride concentration was determined using standard enzymatic methods. Reagents, standards and calibrators were purchased from Pointe Scientific (Lincoln Park, MI). The interassay coefficients of variation are less than 4% and intraassay coefficients of variation are less than 2%. Concentrations of total nitrate and nitrite (NOx) were measured in serum samples by a chemiluminescence method. Serum samples (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution) were filtered through ultrafilter (PL-10 Ultrafree MC centrifugal filter units; Millipore Corp., Bedford, MA) and centrifuged for 1 h at 2500 × g. After removing high-molecular proteins, the filtered serum sample was injected into the reaction chamber of the NO analyzer, and refluxed in glacial acetic acid containing sodium iodide. Nitrate and nitrite in the serum were reduced to NO, which, after reacting with ozone were quantified by a chemiluminescence detector (NOA 280i, Sievers Instruments, Boulder, CO). The concentration was determined in comparison to a sodium nitrate standard calibration curve.
10 dilution) were filtered through ultrafilter (PL-10 Ultrafree MC centrifugal filter units; Millipore Corp., Bedford, MA) and centrifuged for 1 h at 2500 × g. After removing high-molecular proteins, the filtered serum sample was injected into the reaction chamber of the NO analyzer, and refluxed in glacial acetic acid containing sodium iodide. Nitrate and nitrite in the serum were reduced to NO, which, after reacting with ozone were quantified by a chemiluminescence detector (NOA 280i, Sievers Instruments, Boulder, CO). The concentration was determined in comparison to a sodium nitrate standard calibration curve.Cytokine enzyme-linked immunosorbent assays (ELISAs)
IL-6, IL-8, and TNF-alpha (TNF-α) protein levels were measured in plasma of 9 participants using eBioscience Platinum ELISA kits according to manufacturer's instructions. Plasma of two participants was omitted due to hemolysis. Briefly, after a prewash of the wells with PBS + 1% Tween-20 (wash buffer), cytokine standards or plasma diluted with an equal volume of a sample diluent for IL-8 and TNF-α or assay buffer (PBS + 1% Tween-20 + 10% bovine serum albumin) for IL-6. The appropriate biotin-conjugated antibody was diluted in the assay buffer and incubated with the standard or samples for 2 hours on a microplate shaker at 100 rpm at RT. Following 4 washes with the wash buffer, diluted streptavidin–HRP in the assay buffer was incubated in standard and sample wells for 1 hour on a microplate shaker. After washing unbound streptavidin–HRP 4 times with the wash buffer, a tetramethyl–benzidine substrate solution is added to wells. After 10 min, a 1 M phosphoric acid stop solution is added to stop the enzymatic color development in all samples. Absorbance was read at 450 nm for the primary wavelength with a 620 nm reference wavelength on a VersaMax microplate reader (Molecular Devices; Sunnyvale, CA). Interassay coefficients of variation were between 5 and 8% and intraassay coefficients of variation were between 3 and 6%. Grossly hemolyzed samples were excluded from analysis.IκB-α protein expression in peripheral blood mononuclear cells (PBMCs)
PBMCs were isolated from Na-EDTA tubes according to methods described by Aljada et al., 2004.19 After cells were washed with Hank's buffered saline solution, they were lysed with a whole cell lysis buffer (Millipore; Temecula, CA). Total protein concentrations in PBMC lysates were measured by the bicinchoninic acid protein assay (Pierce; Rockland, IL). Equal amounts of protein (40 μg) were then loaded onto 10% gradient gels (Amersham Biosciences, Uppsala, Sweden) and electrophoresed at 100 V. Gels were transferred to a nitrocellulose membrane using the manufacturer's suggested protocol. Membranes were blocked using 5% (w/v) bovine serum albumin in Tris-buffered saline containing 0.001% (v/v) Tween-20 before probing with rabbit polyclonal IκB-α (C-15) antibody (Santa Cruz Biotechnology; Santa Cruz, CA) overnight at 4 °C. After washing the unbound primary antibody, the HRP-conjugated goat anti-rabbit secondary antibody was incubated with the membrane for 2 h at RT. Final chemiluminescence detection was based on an ECL+ kit (Amersham Biosciences, Uppsala, Sweden) as per the manufacturer protocol on a BioRad ChemiDoc XRS (BioRad; Hercules, CA). Membranes were then stripped and reprobed with a mouse monoclonal antibody for beta-actin (Abcam; Cambridge, MA) to ensure equal protein loading. Protein expression was quantified using densitometry on digitized images with the QuantityOne software (BioRad).Statistics
Data were expressed as mean ± standard deviation. Because this is a cross-over design, we used the paired t-test to compare postprandial biomarkers for each time point. To assess the overall difference, we also use the linear mixed effects model to compare postprandial biomarkers over time. An auto-regressive correlation matrix was used to accommodate the correlation from the same subject. All tests were two sided, and differences were considered significant at P < 0.05. SAS 9.1.3 software package was used.Results
Eleven male subjects met both the inclusion and exclusion criteria and were enrolled and completed the study. The characteristics of the subjects are shown in Table 1. The burgers and study protocol were well tolerated and none of the subjects had any adverse event. The baseline PAT score was 2.20 ± 0.36 for participants consuming hamburger only and 2.17 ± 0.57 for those consuming hamburger and avocado. Two hours after the consumption of the test burger, the PAT score was decreased by 27.4 ± 16.6% (2.20 ± 0.36 vs. 1.56 ± 0.21, p = 0.0007) which was prevented when avocado was ingested with the burger (2.17 ± 0.57 vs. 2.08 ± 0.51, NS p = 0.68). The change of PAT score after hamburger versus hamburger + avocado consumption was approaching significant difference (p = 0.052) (Fig. 1B).| N | 11 |
| Age (year) | |
| Mean (SD) | 25.4 (5.7) |
| Race | |
| Asian | 1 |
| Black | 2 |
| Caucasian | 4 |
| Hispanic | 4 |
| BMI (kg m−2) | |
| Mean (SD) | 23.9 (2.3) |
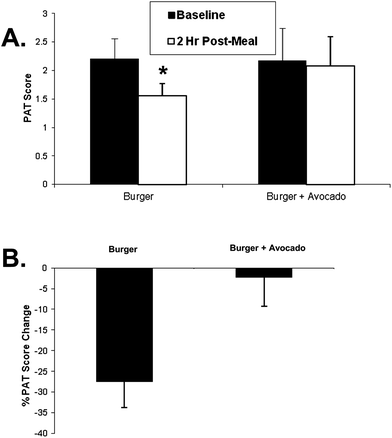 | ||
| Fig. 1 Postprandial PAT changes 2 h after eating a burger or burger + avocado. (A) After eating a burger, PAT scores decreased at 2 h compared to the baseline value (*p < 0.005). However this decrease was prevented by adding avocado to the burger (p = 0.68). (B) Two hour postprandial PAT score changed from baseline after eating a burger or burger + avocado (p = 0.052). Data represent mean ± SEM for 11 subjects (*p < 0.05 from baseline). | ||
The serum nitrate and nitrite concentrations showed a trend to decrease starting at 1 hour post-consumption of hamburger and continued a nonsignificant decrease throughout the following 5 hours. The addition of avocado to hamburger did not change the decrease of postprandial serum nitrite and nitrate levels at any time point (Fig. 2).
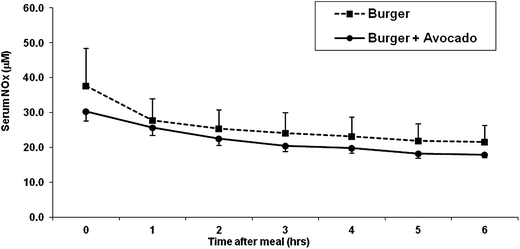 | ||
| Fig. 2 Total serum NOx (nitrate and nitrite) concentrations after eating a burger or burger + avocado. After eating a burger, serum NOx concentration showed a trend to decrease in both burger and burger plus avocado groups. Data represent mean ± SEM for 11 subjects. | ||
Postprandial plasma IL-6 levels increased by ∼70% at 4 hours with burger only but this increase was attenuated when avocado was ingested with the burger despite the added fat and calories from the avocado (Fig. 3A). Neither burger nor burger + avocado affected the postprandial plasma TNF-alpha levels (Fig. 3B). Plasma IL-8 protein was also measured, but levels were below ELISA detection limits in plasma (data not shown).
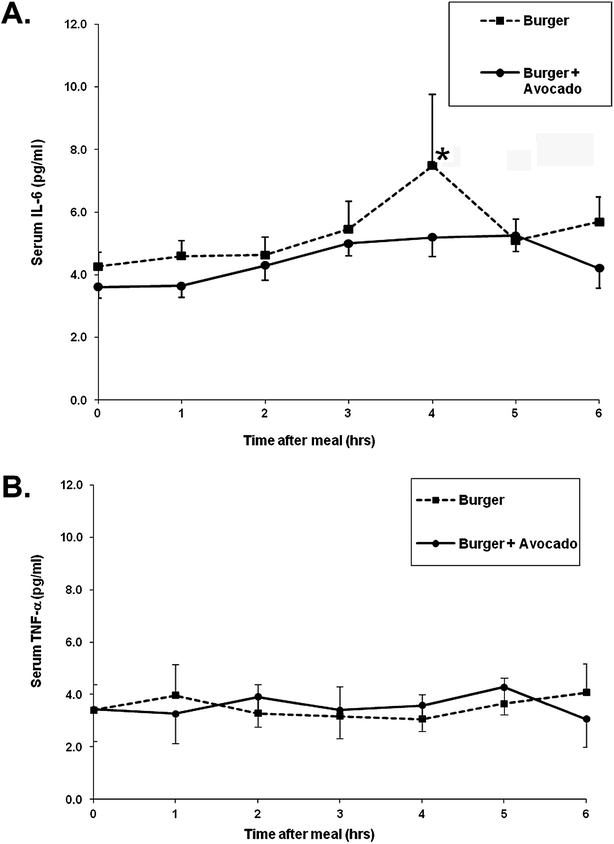 | ||
| Fig. 3 Effects of a burger with or without avocado on postprandial plasma cytokines. (A) Increases in plasma IL-6 levels peak at 4 h following burger consumption, but this increase was attenuated when avocado was added to the burger. (B) Plasma TNF-α protein levels were not affected by either meal. Data represent mean ± SEM for 9 subjects/time point (*p < 0.05 from baseline). | ||
To further investigate the postprandial effect of avocado on inflammation, PBMCs were isolated from postprandial blood samples and IκB-α protein concentration was analyzed as an inverse marker of stimulation of NFκB. NFκB is one of the most important transcription factors in the induction of inflammatory processes.20 NFκB is located in the cytoplasm in an inactive form bound to IκB-α. Upon stimulation IκB-α is phosphorylated and NFκB is released and translocated to the nucleus. IκB-α undergoes ubiquination and degradation via proteasome activity. Therefore stimulation of NFκB is associated with a decrease in IκB-α concentration. Following the burger consumption, IκB-α protein levels decreased in PBMC lysates, suggesting a NFκB pathway activation. However, when avocado was added to the burger, IκB-α protein levels were only slightly elevated. By 3 hours, IκB-α protein was significantly preserved after the consumption of the burger + avocado compared to the burger alone (131% vs. 58%, p = 0.03), suggesting that avocado inhibited peripheral inflammation via the NFκB pathway (Fig. 4).
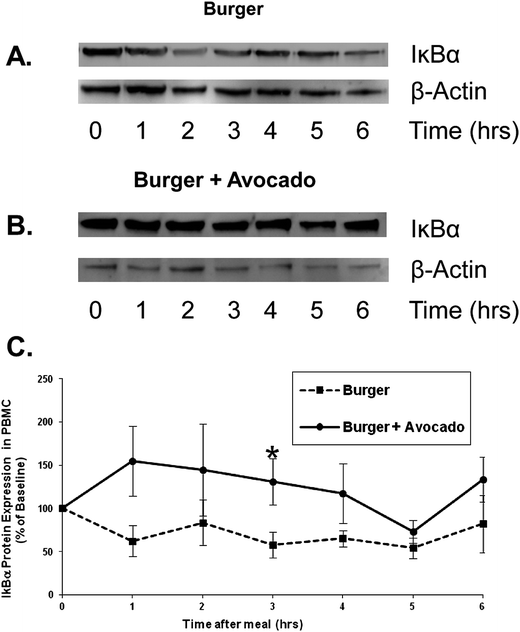 | ||
| Fig. 4 Avocado inhibits NFκB activation in peripheral blood mononuclear cells following a burger meal. Protein levels of IκB-α, the endogenous inhibitor of the NFκB pathway, were determined by Western blots. Representative Western blots showing IκB-α protein degradation following consumption of the burger (A) vs. preservation of IκB-α when avocado was added to the burger (B). (C) Normalized values are expressed as a percentage of the baseline value. Preservation of IκB-α protein by avocado was most pronounced at 3 h post-meal (*p < 0.05 burger vs. burger + avocado). Data represent mean ± SEM for 9 subjects. | ||
There was a significant increase in the postprandial concentration of triglycerides in blood from participants consuming the burger at hours 2, 3 and 4. The level peaked at hour 3, remained high at the end of hour 4 and trended down at hour 5. Addition of avocado did not increase the triglyceride level any further (Fig. 5).
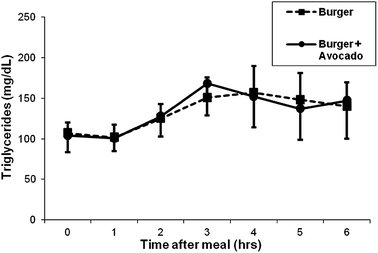 | ||
| Fig. 5 Effects of a burger with or without avocado on postprandial triglyceride. Triglyceride increased following burger consumption, but there was no additional increase by the addition of avocado. Data represent mean ± SEM for 11 subjects (*p < 0.05 from baseline). | ||
Discussion
The presented results from this small pilot study demonstrated for the first time that the addition of avocado to a high fat meal was able to attenuate the postprandial vasoconstrictive effect of a high fat diet. Potential mechanisms for the health beneficial effects may be related to an increase in plasma concentration of the monounsaturated fatty acid oleic acid as shown by Pieterse et al.21 They recently examined the effects of avocados as part of an energy-restricted diet on weight loss, serum lipids and vascular function in overweight and obese subjects randomly assigned to consume energy-restricted diets with total energy intakes of 30% fat, 55% carbohydrates and 15% protein. The experimental group consumed 200 g per day of avocado (30.6 g fat), which was substituted with 30 g dietary fat in the control group. While the avocado-enriched diet had no effect on serum lipids, a significant increase in plasma oleic acid was observed. Partial replacement of complex digestible carbohydrates with monounsaturated fatty acids (avocado as one of its main sources) in the diet of patients with NIDDM also favorably improved both lipid profiles and glycemic control.15While the avocado is an increasingly popular fruit rich in monounsaturated fats, most studies on the benefits of monounsaturated fats have focused on nuts and olive oil with inconsistent outcomes. A study by Cortes et al. demonstrated that the addition of walnut was associated with postprandial vasodilation in patients with hypercholesterolemia, whereas olive oil was associated with vasoconstriction. Both did not show an effect in healthy participants.22 However, the combination of red wine and green olive oil led to vasodilation, while there was no benefit from the addition of white wine and olive oil.23
Chronic inflammation may play a role in the development of atherosclerosis and cardiovascular diseases. Previous epidemiological studies have associated IL-6 with atherosclerosis and increased risk for cardiovascular diseases.24,25 High-fat meals have been shown to influence plasma inflammatory biomarkers such as IL-6, TNF-α, and CRP.26–28 Cytokines, through inhibition of eNOS and the reduction of nitric oxide formation, effect blood vessel constriction.3,4 Foods high in antioxidant and anti-inflammatory phytochemicals, such as tomatoes and strawberries, were shown to inhibit these biomarkers.29,30 Results presented here demonstrated that the addition of avocado, rich in monounsaturated fatty acids and antioxidants, attenuated the postprandial increases in plasma IL-6 induced by a high-fat meal in healthy individuals. This supports the hypothesis that avocado, although energy rich, may provide cardioprotection.
The NFκB signaling pathway is a key immune and inflammatory response pathway underlying atherosclerosis and cardiovascular diseases.31 High-fat meals were shown to induce postprandial NFκB in PBMCs which could be inhibited by the consumption of red wine.32 Meals enriched with butter, but not olive oil or walnut, induced NFκB in PBMCs, suggesting that the monounsaturated fatty acids in walnut and olive oil may be beneficial in preventing postprandial inflammation.33 Another study by Perez-Martinez, 2007, demonstrated that consumption of a Mediterranean diet rich in olive oil did not increase NFκB stimulation compared to the consumption of a Western diet.34
Polyphenols from fruits, vegetables, tea and cocoa have been proposed to improve the endothelial function through nitric oxide (NO) mediated relaxation.35 However, we did not find any change in NO concentration in the serum collected following the burger + avocado intervention compared to that following the burger intervention. Other potential mechanisms regulating the endothelial function may have been involved. For example the polyphenol-induced endothelium-dependent relaxation also involves the endothelium-derived hyperpolarizing factor, besides NO, in several types of arteries.35 Red wine polyphenols have been shown to prevent endothelin 1 induced vascular O2˙− production by reducing overexpression of p47phox and the subsequent increased NADPH oxidase activity, leading to improvement in endothelial function.36 Resveratrol, a polyphenol from grape, was shown to inhibit uterine contraction by blocking external Ca2+ influx induced by PGF2α and high K+ concentrations.37 Avocado may also change the fatty acid profile leading to the alteration of, for example, the fatty acid metabolite prostacyclin (PGI2).38 In summary it appears more likely that avocado may act through other factors influencing the endothelial function other than NO mediated relaxation.
Results from the present pilot study demonstrated that the common culinary practice of adding 1/2 of an avocado (68 g) to a hamburger patty may reduce pro-inflammatory and vasoconstrictory effects of a postprandial challenge with meat patty. In summary we demonstrated that in healthy participants the consumption of 250 g of beef led to a significant decrease in vascular reactivity, increase of serum IL-6 and NFκB activation in PBMCs. The popular practice of adding a slice of avocado to various meat sandwiches was able to reduce these symptoms and may have cardiovascular health benefits. Larger intervention studies are needed to confirm these results.
Acknowledgements
None of the authors had any conflict of interest at the time the research was done. Z. Li, S.M. Henning and D. Heber designed the research and wrote the paper; Y. Zhang, A. Wong, A. Jones, A. Zerlin, G. Thames, and S. Bowerman conducted the research and C.H. Tseng performed statistical analysis. All authors read and approved the final manuscript. This study was supported by the Hass Avocado Board.References
- R. Ross, The pathogenesis of atherosclerosis: a perspective for the 1990s, Nature, 1993, 362, 801–809 CrossRef CAS.
- H. J. Dorman, O. Bachmayer, M. Kosar and R. Hiltunen, Antioxidant properties of aqueous extracts from selected lamiaceae species grown in Turkey, J. Agric. Food Chem., 2004, 52, 762–770 CrossRef CAS.
- J. A. Vita, Endothelial function, Circulation, 2011, 124, e906–e912 CrossRef.
- C. Zhang, The role of inflammatory cytokines in endothelial dysfunction, Basic Res. Cardiol., 2008, 103, 398–406 CrossRef CAS.
- H. Sies, W. Stahl and A. Sevanian, Nutritional, dietary and postprandial oxidative stress, J. Nutr., 2005, 135, 969–972 CAS.
- I. Staprans, D. A. Hardman, X. M. Pan and K. R. Feingold, Effect of oxidized lipids in the diet on oxidized lipid levels in postprandial serum chylomicrons of diabetic patients, Diabetes Care, 1999, 22, 300–306 CrossRef CAS.
- F. Ursini, A. Zamburlini, G. Cazzolato, M. Maiorino, G. B. Bon and A. Sevanian, Postprandial plasma lipid hydroperoxides: a possible link between diet and atherosclerosis, Free Radical Biol. Med., 1998, 25, 250–252 CrossRef CAS.
- M. J. Williams, W. H. Sutherland, M. P. McCormick, S. A. de Jong, R. J. Walker and G. T. Wilkins, Impaired endothelial function following a meal rich in used cooking fat, J. Am. Coll. Cardiol., 1999, 33, 1050–1055 CrossRef CAS.
- J. Kanner and T. Lapidot, The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants, Free Radical Biol. Med., 2001, 31, 1388–1395 CrossRef CAS.
- S. Kubow, Routes of formation and toxic consequences of lipid oxidation products in foods, Free Radical Biol. Med., 1992, 12, 63–81 CrossRef CAS.
- B. Halliwell, K. Zhao and M. Whiteman, The gastrointestinal tract: a major site of antioxidant action?, Free Radical Res., 2000, 33, 819–830 CrossRef CAS.
- J. G. Rodriguez-Carpena, D. Morcuende, M. J. Andrade, P. Kylli and M. Estevez, Avocado (Persea Americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties, J. Agric. Food Chem., 2011, 59, 5625–5635 CrossRef CAS.
- Q. Y. Lu, Y. Zhang and Y. Wang, et al. California Hass avocado: profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas, J. Agric. Food Chem., 2009, 57, 10408–10413 CrossRef CAS.
- D. M. Colquhoun, D. Moores, S. M. Somerset and J. A. Humphries, Comparison of the effects on lipoproteins and apolipoproteins of a diet high in monounsaturated fatty acids, enriched with avocado, and a high-carbohydrate diet, Am. J. Clin. Nutr., 1992, 56, 671–677 CAS.
- I. Lerman-Garber, S. Ichazo-Cerro, J. Zamora-Gonzalez, G. Cardoso-Saldana and C. Posadas-Romero, Effect of a high-monounsaturated fat diet enriched with avocado in NIDDM patients, Diabetes Care, 1994, 17, 311–315 CAS.
- L. R. Lopez, A. C. Frati Munari and B. C. Hernandez Dominguez, et al. Monounsaturated fatty acid (avocado) rich diet for mild hypercholesterolemia, Arch. Med. Res., 1996, 27, 519–523 Search PubMed.
- N. M. Hamburg, M. J. Keyes and M. G. Larson, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study, Circulation, 2008, 117, 2467–2474 CrossRef.
- P. O. Bonetti, G. W. Barsness and P. C. Keelan, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease, J. Am. Coll. Cardiol., 2003, 41, 1761–1768 CrossRef.
- A. Aljada, P. Mohanty and H. Ghanim, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect, Am. J. Clin. Nutr., 2004, 79, 682–690 CAS.
- G. Sethi, B. Sung and B. B. Aggarwal, Nuclear factor-kappaB activation: from bench to bedside, Exp. Biol. Med., 2008, 233, 21–31 CAS.
- Z. Pieterse, J. C. Jerling and W. Oosthuizen, et al. Substitution of high monounsaturated fatty acid avocado for mixed dietary fats during an energy-restricted diet: effects on weight loss, serum lipids, fibrinogen, and vascular function, Nutrition, 2005, 21, 67–75 CrossRef CAS.
- B. Cortes, I. Nunez and M. Cofan, et al. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function, J. Am. Coll. Cardiol., 2006, 48, 1666–1671 CrossRef CAS.
- K. Karatzi, C. Papamichael and E. Karatzis, et al. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: a synergistic effect of components of the Mediterranean diet, J. Am. Coll. Nutr., 2008, 27, 448–453 CAS.
- M. B. Lobbes, E. Lutgens and S. Heeneman, et al. Is there more than C-reactive protein and fibrinogen? The prognostic value of soluble CD40 ligand, interleukin-6 and oxidized low-density lipoprotein with respect to coronary and cerebral vascular disease, Atherosclerosis, 2006, 187, 18–25 CrossRef CAS.
- I. Tzoulaki, G. D. Murray, A. J. Lee, A. Rumley, G. D. Lowe and F. G. Fowkes, C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study, Circulation, 2005, 112, 976–983 CrossRef CAS.
- P. Lundman, S. Boquist and A. Samnegard, et al. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations, Nutr., Metab. Cardiovasc. Dis., 2007, 17, 195–202 CrossRef CAS.
- F. Nappo, K. Esposito and M. Cioffi, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals, J. Am. Coll. Cardiol., 2002, 39, 1145–1150 CrossRef CAS.
- C. Payette, P. Blackburn and B. Lamarche, et al. Sex differences in postprandial plasma tumor necrosis factor-alpha, interleukin-6, and C-reactive protein concentrations, Metabolism, 2009, 58, 1593–1601 CrossRef CAS.
- B. Burton-Freeman, J. Talbot, E. Park, S. Krishnankutty and I. Edirisinghe, Protective activity of processed tomato products on postprandial oxidation and inflammation: a clinical trial in healthy weight men and women, Mol. Nutr. Food Res., 2012, 56, 622–631 CAS.
- I. Edirisinghe, K. Banaszewski and J. Cappozzo, et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin, Br. J. Nutr., 2011, 106, 913–922 CrossRef CAS.
- C. Monaco and E. Paleolog, Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis, Cardiovasc. Res., 2004, 61, 671–682 CrossRef CAS.
- L. M. Blanco-Colio, M. Valderrama and L. A. varez-Sala, et al. Red wine intake prevents nuclear factor-kappaB activation in peripheral blood mononuclear cells of healthy volunteers during postprandial lipemia, Circulation, 2000, 102, 1020–1026 CrossRef CAS.
- Y. Jimenez-Gomez, J. Lopez-Miranda and L. M. Blanco-Colio, et al. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men, Atherosclerosis, 2009, 204, e70–e76 CrossRef CAS.
- P. Perez-Martinez, J. Lopez-Miranda and L. Blanco-Colio, et al. The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor kappaB activation in peripheral blood mononuclear cells from healthy men, Atherosclerosis, 2007, 194, e141–e146 CrossRef CAS.
- V. B. Schini-Kerth, C. Auger, J. H. Kim, N. Etienne-Selloum and T. Chataigneau, Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF, Pflugers Arch., 2010, 459, 853–862 CrossRef CAS.
- R. Lopez-Sepulveda, M. Gomez-Guzman and M. J. Zarzuelo, et al. Red wine polyphenols prevent endothelial dysfunction induced by endothelin-1 in rat aorta: role of NADPH oxidase, Clin. Sci., 2011, 120, 321–333 CrossRef CAS.
- S. M. Hsia, K. L. Wang and P. S. Wang, Effects of resveratrol, a grape polyphenol, on uterine contraction and Ca(2)+ mobilization in rats in vivo and in vitro, Endocrinology, 2011, 152, 2090–2099 CrossRef CAS.
- C. Skogastierna, L. Luksha, K. Kublickiene, E. Eliasson, A. Rane and L. Ekstrom, Beneficial vasoactive endothelial effects of fluvastatin: focus on prostacyclin and nitric oxide, Heart Vessels, 2011, 26, 628–636 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
