Effects of Korean red ginseng as an adjuvant to bile acids in medical dissolution therapy for gallstones: a prospective, randomized, controlled, double-blind pilot trial
Jun Kyu
Lee
,
Hyoun Woo
Kang
,
Jae Hak
Kim
,
Yun Jeong
Lim
,
Moon-Soo
Koh
and
Jin Ho
Lee
*
Department of Internal Medicine, Dongguk University Ilsan Hospital, College of Medicine, Dongguk University, Korea
First published on 1st October 2012
Abstract
Although ginseng, the root of Panax quinquefolium and P. ginseng, was reported to have anti-cholelithogenic effects in animal experiments, there have, to date, been no human studies. We conducted this prospective, controlled, double-blind pilot trial to evaluate the safety and efficiency of Korean red ginseng (KRG), the steamed root of P. ginseng C.A. Meyer. Twenty eight consecutive patients were randomized to receive either KRG (7.5 g divided into three daily doses) or a placebo as an adjuvant to the standard regimen of bile acids for gallstones (500 mg of chenodeoxycholic acid and 500 mg of ursodeoxycholic acid divided into three daily doses) for 24 weeks. No case of serious adverse reaction occurred in both groups. Although the decrease in stone burden was larger in the KRG group (3.4 ± 0.6 ml3) than in the placebo group (2.3 ± 1.1 ml3), it did not reach statistical significance (p = 0.09). Also there were no differences in the rate of complete dissolution, subjective improvement in symptoms, and the rate of cholecystectomy due to worsening pain or the development of complications and changes in laboratory tests before and after treatment. In conclusion, the addition of KRG as an adjuvant was safe for patients undergoing bile acid dissolution therapy for gallstones although it did not affect the results. Large-scaled trials to optimize regimens are expectantly needed.
Introduction
Gallstone disease is a common medical condition, the prevalence of which ranges from 10 to 15% among the global population, and may complicate cholecystitis, cholangitis and pancreatitis.1 Also, gallstones are regarded to be causally attributable to the development of gallbladder cancer and reported to be associated with decreased rates of mortality.2,3 The pathogenesis of cholesterol gallstones, which is most common in developed countries throughout the world, is understood clearly. The supersaturation and nucleation of cholesterol in the gallbladder are essential to the formation of cholesterol gallstones. Bile acid therapy which inhibits these steps is the current standard of medical dissolution for gallstones.4,5 However, since the rate of dissolution is unsatisfactory and recurrence is frequent, a breakthrough is needed urgently.6Ginseng is the root of the perennial herbs of Panax quinquefolium and P. ginseng which contain a series of tetracyclic triterpenoid saponins (ginsenosides) as active ingredients.7 Recently, it was shown that ginseng could reduce bile flow and secretion of total lipids and cholesterol in animal experiments by which an anti-cholelithogenic effect may be expected.8,9 There have been, however, no clinical trials to assess these effects in humans. We have designed this prospective, randomized and controlled double-blind pilot trial to evaluate the safety and efficiency of Korean red ginseng (KRG), the steamed root of P. ginseng C.A. Meyer, which was added as an adjuvant to the standard chenodeoxycholic acid (CDCA) and ursodeoxycholic acid (UDCA) regimen.
Materials and methods
Study population
Patients over 18 years of age with biliary colic (defined as more than one episode of right upper quadrant or epigastric pain lasting more than 30 min) and diagnosed with ultrasonography-proven gallstones were enrolled consecutively at Dongguk University Ilsan Hospital from May 2010 to April 2011. Exclusion criteria were as follows: the preference of surgical treatment, gallstones >15 mm in diameter or surface calcification, body mass index ≥35 kg m−2, severe and frequent pain, non-functioning gallbladder, previous bile acid treatment, abnormality of liver function tests over 4 weeks, alcohol or drug abuse and pregnant or lactating women. This trial followed guidelines of the Declaration of Helsinki and Tokyo for humans. The Institutional Review Board approved this trial and written informed consents for voluntary participation were obtained from all of the patients before they entered the study.Study design
After inclusion, patients were randomized to receive either 7.5 g of KRG (Jeongkwanjang Red Ginseng Powder Capsule®, Korea Red Ginseng Corporation, Daejeon, Korea) or placebo as an adjuvant to CDCA 500 mg plus UDCA 500 mg divided into three daily doses for 24 weeks.Each patient was followed-up at 4, 12 and 24 weeks for an interview, a physical examination and laboratory tests. At each visit, subjective improvement in symptoms was evaluated by the self-reported visual analog scale. At 24 weeks, the changes in characteristics, the maximum diameter and number of gallstones were assessed with ultrasonography. Patient compliance was assessed by capsule count.
Outcomes of interest
Primary outcomes of interest were the occurrence of serious adverse reaction and decrease in stone burden. A serious adverse reaction was defined as any noxious, unintended and undesired effect by which the administration of drugs needed to be stopped. Stone burden was defined as the sum of the volume of the five largest stones. The volume of each stone was calculated as for a sphere after measuring the diameter.10 Secondary outcomes of interest were as follows: the rate of complete dissolution, subjective improvement in symptoms, the rate of cholecystectomy due to worsening pain or development of complications, and changes in laboratory tests before and after treatment.Statistical analysis
The differences in categorical variables were analyzed using the chi-square test with Yates' correction or Fisher's exact test, as applicable. Mean values were expressed as the mean ± standard errors (SE) and compared using the Student's t test. Differences were considered statistically significant when p < 0.05. Data were analyzed using R-software (http://www.R-project.org).Results
A total of 32 patients were enrolled during the study period and were randomized to either the KRG or placebo group. Four patients dropped out due to a failure to attend follow-up appointments or a preference for surgical treatment (3 and 1 patients, respectively). The final analysis was performed with the remaining 28 patients and there were 14 in each group. Baseline characteristics showed no differences between groups (Table 1).| Korean red ginseng (n = 14) | Placebo (n = 14) | p | |
|---|---|---|---|
| a Values other than those in the rows of gender and multiplicity of stone are expressed as mean ± standard errors. b The sum of the volumes of the largest five stones calculated as for spheres. | |||
| Age (years) | 63 ± 2.8 | 60.4 ± 5.5 | 0.10 |
| Gender (M/F) | 4/10 | 6/8 | 0.21 |
| Body mass index (kg m−2) | 24.3 ± 2.4 | 21.4 ± 1.4 | 0.20 |
| Stone burdenb, initial (ml3) | 10.1 ± 1.7 | 9.7 ± 1.0 | 0.24 |
| Multiplicity of stone, no. (%) | 5 (35.7) | 7 (50.0) | 0.65 |
| Total bilirubin (mg dL−1) | 0.76 ± 0.12 | 0.74 ± 0.10 | 0.86 |
| Aspartate aminotransferase (IU L−1) | 48.0 ± 7.9 | 41.4 ± 4.4 | 0.48 |
| Alanine aminotransferase (IU L−1) | 51.6 ± 8.7 | 43.4 ± 4.3 | 0.40 |
| Fasting glucose (mg dL−1) | 121.8 ± 12.3 | 116.8 ± 9.7 | 0.07 |
| Total cholesterol (mg dL−1) | 228.7 ± 10.6 | 214.9 ± 10.4 | 0.39 |
No case of serious adverse reaction occurred in the whole study population (Table 2). Although the decrease in stone burden was larger in the KRG group (3.4 ± 0.6 ml3) than in the placebo group (2.3 ± 1.1 ml3), it did not reach statistical significance (p = 0.09). The complete dissolution of stones was achieved in 8 (57.1%) patients from the KRG group and in 6 (42.9%) from the placebo group out of 14 patients respectively (p = 0.26). A total of 11 (78.6%) patients reported subjective improvement in symptoms in the KRG group and 10 (71.4%) in the placebo group (p = 0.20). Cholecystectomy was performed for 1 (7.1%) patient from each group respectively (p = 0.99). There were no meaningful changes in laboratory tests. A representative case of successful gallstone dissolution is depicted in Fig. 1.
| Korean red ginseng (n = 14) | Placebo (n = 14) | p | |
|---|---|---|---|
| a Values other than those in the rows of rate of complete dissolution, subjective improvement in symptoms, rate of cholecystectomy and adverse reactions are expressed as mean ± standard errors. b The sum of volumes of the largest five stones calculated as for spheres. | |||
| Primary outcomes of interest | |||
| Serious adverse reactions, no. (%) | 0 (0) | 0 (0) | 0.99 |
| Decrease in stone burdenb | 3.4 ± 0.6 | 2.3 ± 1.1 | 0.09 |
| Secondary outcomes of interest | |||
| Rate of complete dissolution, no. (%) | 8 (57.1) | 6 (42.9) | 0.26 |
| Subjective improvement no. (%) | 11 (78.6) | 10 (71.4) | 0.20 |
| Rate of cholecystectomy, no. (%) | 1 (7.1) | 1 (7.1) | 0.99 |
| Changes in laboratory tests before and after treatment | |||
| Total bilirubin (mg dL−1) | −0.10 ± 0.07 | −0.05 ± 0.04 | 0.45 |
| Aspartate aminotransferase (IU L−1) | −4.0 ± 2.2 | +9.8 ± 3.6 | 0.39 |
| Alanine aminotransferase (IU L−1) | −7.9 ± 4.3 | +7.6 ± 4.3 | 0.46 |
| Fasting glucose (mg dL−1) | −20.0 ± 6.3 | −16.7 ± 3.3 | 0.70 |
| Total cholesterol (mg dL−1) | −22.3 ± 4.5 | −19.2 ± 3.8 | 0.84 |
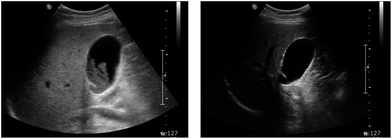 | ||
| Fig. 1 Ultrasonographic images of before (left) and after (right) medical dissolution therapy for gallstones. A 28 year-old female patient was treated with a regimen in which Korean red ginseng was added to the bile acids and gallstones were dissolved completely after 24 weeks. | ||
Discussion
This study showed that KRG could be safely added as an adjuvant to the standard CDCA plus UDCA regimen for gallstones although it did not affect the results of dissolution therapy. Currently, laparoscopic cholecystectomy is a favored option for patients with gallstones that are symptomatic or complicated, especially when pigmented stones are expected. Although the incidence is not high, however, cholecystectomy cannot be free from complications such as bile duct injury causing morbidities and increased medical costs.11 For the very old or debilitated patients, high surgical risks might disturb surgery itself. Most importantly, the incidence of cholesterol stones is rapidly increasing, notably among young ages, with industrialization and the westernization of life styles in Asian countries where pigmented gallstones related with clonorchiasis or salmonellosis have become increasingly prevalent.12–14 Medical dissolution therapy deserves to be highlighted again because it can be considered advantageous compared to surgery for cholesterol stones. Similar changes in disease patterns are expected in many developing countries outside of Asia.Currently, CDCA, UDCA, or the combination of both is used for the medical dissolution of gallstones.10,15–17 However, treatment outcomes are not satisfactory and the rates of complete dissolution were reported to be 18.2% by high-dose CDCA, 37.3% by high-dose UDCA and 62.8% by the combination of both according to a meta-analysis.18 Since a high cholesterol concentration in bile is essential for the formation of cholesterol gallstones, a number of drugs modulating cholesterol metabolism are under active investigation, e.g. statins, competitive inhibitors of HMG-CoA which determine the rate-limiting step in cholesterol biosynthesis; ezetimibe, a specific inhibitor of the intestinal cholesterol transporter protein Niemann-Pick C1-like 1 (NPC1L1); and liver-specific agonists/antagonists of liver X receptor of oxysterol (LXR) or bile acid receptor farnesoid X (FXR).19–22 However, besides adverse reactions such as liver toxicity or myopathy,23 frequent recurrence of stones even after the successful induction of dissolution is the major drawback of medical therapy as cholelithogenic conditions are unlikely to be changed even though gallstones are dissolved. Therefore, to prevent recurrence would be as important as induction, which is supported by the finding that the recurrence rate reached 61% at the 11th year after successful medical dissolution treatment.24 In developing preventive regimens, herbal medicines which are feasible for long-term maintenance would be excellent alternative candidates. Among them, turmeric root (Curcuma longa), Oregon grape (Mahonia aquifolium) and coin grasses (Lysimachia christinae, Desmodium styracifolium and Glechoma hederacea) are noticeable because they have been included in the traditional gallstone remedy of Asian, ayurvedic, and western phytomedicines and anti-cholelithogenic effects were reported in preclinical studies.25 Unfortunately, however, no clinical trials have been conducted yet with these agents.
Ginseng has been used as a tonic for the treatment of a variety of ailments in Asia for thousands of years. Ginseng has a number of pharmacological effects including immunomodulatory, anti-inflammatory and anti-tumor activities and is most promising as a supplement added to the conventional treatment of chronic diseases.26 However, because pharmacokinetic studies are limited on ginseng or ginsenosides, there are concerns about toxicity and drug-to-drug interactions. Although some studies reported the hepato-protective effects of ginseng,9,27 the combination with drugs acting on the hepatobiliary system is of special concern since ginsenosides are partly metabolized by hepatic P450 enzymes. As this was the first human study about the combination with bile acids as far as we know, safety was of the utmost importance and we chose one of the lowest doses (7.5 g per day) used in published trials with KRG in humans for various diseases.28–32 The suboptimal dosage might be a limitation of this pilot study, together with an insufficient number of subjects and a shorter duration compared with previous studies for bile acid therapy against gallstones. However, we believe our findings would serve as a good stepping-stone toward future trials to elucidate the overall efficiency of ginseng against gallstones by providing safety profiles. Particularly, studies regarding the usefulness of ginseng for consolidation after dissolution are highly anticipated.
In conclusion, the addition of KRG as an adjuvant was safe for patients undergoing bile acid dissolution therapy for gallstones although it did not affect the results. Large-scaled trials to optimize regimens are expectantly needed.
Acknowledgements
This work was supported by the 2010 grant of the Korean Society of Ginseng funded by the Korea Red Ginseng Corporation.References
- R. S. Sandler, J. E. Everhart, M. Donowitz, E. Adams, K. Cronin, C. Goodman, E. Gemmen, S. Shah, A. Avdic and R. Rubin, The burden of selected digestive diseases in the United States, Gastroenterology, 2002, 122, 1500–1511 CrossRef.
- J. Hart, B. Modan and M. Shani, Cholelithiasis in the aetiology of gallbladder neoplasm, Lancet, 1971, 1(7710), 1151–1153 CrossRef CAS.
- C. E. Ruhl and J. E. Everhart, Gallstone disease is associated with increased mortality in the United States, Gastroenterology, 2011, 140, 508–516 CrossRef.
- Y. H. Park, H. Igimi and M. C. Carey, Dissolution of human cholesterol gallstones in stimulated chenodeoxycholate-rich and ursodeoxycholate-rich biles. An in vitro study of dissolution rates and mechanisms, Gastroenterology, 1984, 87, 150–158 CAS.
- J. R. Senior, M. F. Johnson, D. M. DeTurck, F. Bazzoli and E. Roda, In vivo kinetics of radiolucent gallstone dissolution by oral dihydroxy bile acids, Gastroenterology, 1990, 99, 243–251 CAS.
- A. Di Ciaula, D. Q. Wang, H. H. Wang, L. Bonfrate and P. Portincasa, Targets for current pharmacologic therapy in cholesterol gallstone disease, Clin. Gastroenterol., 2010, 39, 245–264 Search PubMed.
- E. Nocerino, M. Amato and A. A. Izzon, The aphrodisiac and adaptogenic properties of ginseng, Fitoterapia, 2000, 71(suppl 1), S1–S5 CrossRef CAS.
- O. M. Salam, S. A. Nada and M. S. Arbid, The effect of ginseng on bile-pancreatic secretion in the rat. Increase in proteins and inhibition of total lipids and cholesterol secretion, Pharmacol. Res., 2002, 45, 349–353 CrossRef.
- X. Cui, T. Sakaguchi, D. Ishizuka, K. Tsukada and K. Hatakeyama, Orally administered ginseng extract reduces serum total cholesterol and triglycerides that induce fatty liver in 66% hepatectomized rats, J. Int. Med. Res., 1998, 26, 181–187 CAS.
- M. L. Petroni, R. P. Jazrawi, P. Pazzi, A. Lanzini, M. Zuin, M. G. Pigozz, M. Fracchia, G. Galatola, V. Alvisi, K. W. Heaton, M. Podda and T. C. Northfield, Ursodeoxycholic acid alone or with chenodeoxycholic acid for dissolution of cholesterol gallstones: a randomized multicentre trial. The British–Italian gallstone study group, Aliment. Pharmacol. Ther., 2001, 15, 123–128 CrossRef CAS.
- J. A. Shea, M. J. Healey, J. A. Berlin, J. R. Clarke, P. F. Malet, R. N. Staroscik, J. S. Schwartz and S. V. Williams, Mortality and complications associated with laparoscopic cholecystectomy. A meta-analysis, Ann. Surg., 1996, 224, 609–620 CrossRef CAS.
- G. W. Youn, S. I. Whang and J. H. Shin, 15 years of experience in laparoscopic cholecystectomy by a single surgeon, Journal of the Korean Surgical Society, 2009, 76, 173–178 CrossRef.
- X. Zhu, S. Zhang and Z. Huang, The trend of the gallstone disease in China over the past decade, Zhonghua Wai Ke Za Zhi, 1995, 33, 652–658 CAS.
- F. Nakayama and H. Miyake, Changing state of gallstone disease in Japan. Composition of the stones and treatment of the condition, Am. J. Surg., 1970, 120, 794–799 CrossRef CAS.
- R. G. Danzinger, A. F. Hofmann, L. J. Schoenfield and J. L. Thistle, Dissolution of cholesterol gallstones by chenodeoxycholic acid, N. Engl. J. Med., 1972, 286, 1–8 CrossRef CAS.
- H. J. Fromm, J. W. Roat, V. Gonzalez, R. P. Sarva and S. Farivar, Comparative efficacy and side effects of ursodeoxycholic and chenodeoxycholic acids in dissolving gallstones. A double-blind controlled study, Gastroenterology, 1983, 85, 1257–1264 CAS.
- H. Y. Mok, G. D. Bell and R. H. Dowling, Effect of different doses of chenodeoxycholic acid on bile-lipid composition and on frequency of side-effects in patients with gallstones, Lancet, 1974, 2(7875), 253–257 CrossRef CAS.
- G. R. May, L. R. Sutherland and E. A. Shaffer, Efficacy of bile acid therapy for gallstone dissolution: a meta-analysis of randomized trials, Aliment. Pharmacol. Ther., 1993, 7, 139–148 CrossRef CAS.
- T. E. Miettinen, T. Kiviluoto, M. Taavitsainen, M. Vuoristo and T. A. Miettinen, Cholesterol metabolism and serum and biliary noncholesterol sterols in gallstone patients during simvastatin and ursodeoxycholic acid treatments, Hepatology, 1998, 27, 649–655 CrossRef CAS.
- F. X. Caroli-Bosc, P. Le Gall, P. Pugliese, B. Delabre, C. Caroli-Bosc, J. F. Demarquay, J. P. Delmont, P. Rampal and J. C. Montet, Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation: epidemiological study in an unselected population, Dig. Dis. Sci., 2001, 46, 540–544 CrossRef CAS.
- H. Uppal, Y. Zhai, A. Gangopadhyay, S. Khadem, S. Ren, J. A. Moser and W. Xie, Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization, Hepatology, 2008, 47, 1331–1342 CrossRef CAS.
- A. Moschetta, A. L. Bookout and D. J. Mangelsdorf, Prevention of cholesterol gallstone disease by FXR agonists in a mouse model, Nat. Med., 2004, 10, 1352–1358 CrossRef CAS.
- R. C. Gillett Jr and A. Norrell, Considerations for safe use of statins: liver enzyme abnormalities and muscle toxicity, Am. Fam. Physician, 2011, 83, 711–716 Search PubMed.
- N. Villanova, F. Bazzoli, F. Taroni, R. Frabboni, G. Mazzella, D. Festi, L. Barbara and E. Roda, Gallstone recurrence after successful oral bile acid treatment. A 12-year follow-up study and evaluation of long-term postdissolution treatment, Gastroenterology, 1989, 97, 726–731 CAS.
- M. M. Moga, Alternative treatment of gallbladder disease, Med. Hypotheses, 2003, 60, 143–147 CrossRef CAS.
- J. M. Lu, Q. Yao and C. Chen, Ginseng compounds: an update on their molecular mechanisms and medical applications, Curr. Vasc. Pharmacol., 2009, 7, 293–302 CrossRef CAS.
- T. C. Jeong, H. J. Kim, J. I. Park, C. S. Ha, J. D. Park, S. I. Kim and J. K. Roh, Protective effects of red ginseng saponins against carbon tetrachloride-induced hepatotoxicity in Sprague Dawley rats, Planta Med., 1997, 63, 136–140 CrossRef CAS.
- T. K. Yun, S. Zheng, S. Y. Choi, S. R. Cai, Y. S. Lee, X. Y. Liu, K. J. Cho and K. Y. Park, Non-organ-specific preventive effect of long-term administration of Korean red ginseng extract on incidence of human cancers, J. Med. Food, 2010, 13, 489–494 CrossRef CAS.
- V. Vuksan, M. K. Sung, J. L. Sievenpiper, P. M. Stavro, A. L. Jenkins, M. Di Buono, K. S. Lee, L. A. Leiter, K. Y. Nam, J. T. Arnason, M. Choi and A. Naeem, Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety, Nutr., Metab. Cardiovasc. Dis., 2008, 18, 46–56 CrossRef.
- J. H. Heo, S. T. Lee, K. Chu, M. J. Oh, H. J. Park, J. Y. Shim and M. Kim, An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease, Eur. J. Neurol., 2008, 15, 865–868 CrossRef.
- J. L. Sievenpiper, M. K. Sung, M. Di Buono, K. Seung-Lee, K. Y. Nam, J. T. Arnason, L. A. Leiter and V. Vuksan, Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies, J. Am. Coll. Nutr., 2006, 25, 100–107 CAS.
- H. Sung, S. M. Kang, M. S. Lee, T. G. Kim and Y. K. Cho, Korean red ginseng slows depletion of CD4 T cells in human immunodeficiency virus type 1-infected patients, Clin. Diagn. Lab. Immunol., 2005, 12, 497–501 CAS.
| This journal is © The Royal Society of Chemistry 2013 |
