 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atmospheric deposition of current use pesticides in the Arctic: Snow core records from the Devon Island Ice Cap, Nunavut, Canada†
Xianming
Zhang
a,
Torsten
Meyer
a,
Derek C. G.
Muir
*b,
Camilla
Teixeira
b,
Xiaowa
Wang
b and
Frank
Wania
a
aDepartment of Physical and Environmental Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto, Ontario M1C 1A4, Canada
bAquatic Contaminants Research Division, Environment Canada, Burlington, Ontario L7R 4A6, Canada. E-mail: derek.muir@ec.gc.ca; Tel: +1-905-319-6921
First published on 7th October 2013
Abstract
Current use pesticides (CUPs) have been detected in the Arctic, even though there are no direct sources and their long range atmospheric transport potential is generally lower than that of legacy pesticides. Data on the deposition of CUPs in the Arctic are required to assess the impact of their global usage and emission. In this study, selected CUPs were measured in the layers of a snow pit sampled on the Devon Ice Cap, Nunavut, Canada. The oldest sampled layers correspond to deposition from the early 1990s. Dacthal and endosulfan sulfate were most frequently detected, with peak deposition fluxes of 1.0 and 0.4 pg cm−2 per year. While endosulfan sulfate was more abundant than its parent compounds in most years, endosulfan (sum of α and β isomers) was predominant in 2003 and 2006, which together with air mass backward trajectories suggests a possible origin from ongoing use in Eurasia. The interannual variation in CUP deposition fluxes could not be explained with annual variations in the extent of air mass origin over agricultural lands, suggesting that other factors, such as the interannual variation in pesticide use, play a role in affecting the long range transport of CUPs to the Arctic. The very high variability in the concentrations of CUPs in the horizontal layers of Arctic ice caps is most plausibly explained by the highly episodic nature of long range atmospheric transport and deposition. While this strong influence of rare events limits the suitability of ice caps as reliable records of historical trends in Arctic contaminant deposition with annual resolution, the presence of concentration peaks in the ice record is proof of the possibility of such transport and deposition.
Environmental impactQuantitative information on the deposition of current use pesticides (CUPs) in the Arctic is important for assessing the impact of their global usage and emission. Snowpack acts as a reservoir for atmospherically deposited organic contaminants in the Arctic and plays an important role in the environmental fate and impact of the contaminants. In this study, using a snow pit sampled on the Devon Ice Cap, Nunavut, Canada, we inferred the atmospheric deposition history of semivolatile organic compounds from the early 1990s to the mid 2000s. Large interannual variations of the deposition fluxes indicate a highly episodic nature of long range atmospheric transport and deposition. |
Introduction
Pesticides are produced in high volumes and are extensively used in tropical and temperate agriculture. Although those in current use (CUPs) tend to be less persistent and bioaccumulative than many of the banned legacy pesticides, they have been detected in samples from the Arctic,1–6 where they have never been produced and are not likely to have ever been used. Transport of semivolatile organic compounds to the Arctic is believed to occur mainly through the atmosphere.7–9 Concerns related to the presence of CUPs in the Arctic arise, because as inherently toxic chemicals they may pose a risk to the local ecosystems.5,10The pesticides can deposit on Arctic surfaces via diffusive gas exchange and together with rain, snow and atmospheric particles.11 Snow, due to its highly porous structure and low prevailing temperatures, can effectively scavenge both gas phase and particle-bound chemicals from the air12 and is thus believed to contribute significantly to the atmospheric deposition of semivolatile contaminants in the Arctic.11 After deposition, the snowpack can act as a reservoir for the contaminants. Depending on the physicochemical properties and facilitated by physical changes in the snow structure during snow ageing and snowmelt, contaminants accumulated in surface snow may be re-emitted to the atmosphere or transferred deeper into aged snowpack and possibly released to ecosystems.13–15 Therefore, snow plays an important role in the environmental fate and impact of atmospherically deposited organic contaminants in the Arctic.16–18
The presence of pesticide residues in snow from remote regions has been used as evidence of global scale long range atmospheric transport for a long time, beginning with the detection of DDT in Antarctic snow more than 40 years ago.19,20 Since then, pesticides have been regularly detected in snow from the Arctic6,16,21 and mid-latitude mountains.22–24 In the 1990s, snow pits dug on the ice caps on Ellesmere Island, Nunavut, Canada;25,26 on Greenland;27,28 and in the Canadian Rocky Mountains29 were used to infer the atmospheric deposition history of semivolatile organic compounds. More recently, similar approaches have been used in Svalbard2,30,31 and Antarctica.32 The Devon Ice Cap has been used to infer the deposition of antimony,33 perfluoroalkyl acids34 and brominated flame retardants.18 Depth profiles of semivolatile organic contaminants in snow pits from Arctic ice caps generally display very high variability between the layers corresponding to different deposition years; also, time trends, if apparent at all, tend not to correlate very well with time trends in the global usage of the analyzed compounds.
While the contaminant concentration in fresh snow directly reflects atmospheric deposition, concentrations in aged snow and firn may be affected by post-depositional processes such as re-volatilization to the air and transfer to deeper firn layers caused by wind-pumping, seasonal temperature increase and melt.18,35,36 The effect of such post-depositional processes depends on compound and snow properties; volatile compounds in permeable snow are more likely to re-volatilize while water soluble compounds are more likely to move with the meltwater.13,15 Even in Arctic ice caps at high altitude, where seasonal snow melt is limited, the relocation of chemicals in the snowpack due to post-depositional processes makes it highly uncertain to retrieve the atmospheric deposition history at a very high temporal resolution. However, layers with an approximately annual resolution can be inferred from the chemical vertical profiles measured in snow and ice cores.18,29,32
In this study, snow cores were sampled from the Devon Ice Cap, Nunavut, Canada in 2008 and analyzed for a suite of CUPs in commerce in North America and Europe (Table S1†). The CUPs were selected based on their known use and on their analyzability by gas chromatography-negative chemical ionization mass spectrometry (GC-NCI-MS). A similar suite was analyzed by Ruggirello et al.31 Whereas results for brominated flame retardants have been presented previously,18 we present here the concentrations and historical deposition fluxes of selected CUPs. With the vertical concentration profiles of the chemicals in the snow core, we aim to assess the historical trends of the chemicals deposited on the Arctic surface and importance of source regions.
Methods
Sample collection
The procedures for sample collection have been described by Meyer et al. 2012.18 Briefly, in May 2008, a snow pit was sampled from the Devon Ice Cap, Nunavut (75°20.4′ N and 82°40.2′ W) (Fig. 1) to a depth of 7 m. The pit was located several kilometers upwind from the nearest temporary research site of the CryoSat-2 line37 at ∼1800 m above sea level. Single bulk samples covering the entire vertical stretch were taken vertically at 50 cm intervals along the face of the pit using 4 L polypropylene bottles. The sample representing the depth range from 3 m to 3.5 m was not available for analysis due to the loss in the field. Additional smaller samples were taken at 10 cm intervals for density and ion analysis. All the samples were shipped frozen by air-freight to the Canada Centre for Inland Waters (CCIW), Burlington, Canada and kept at −20 °C until analysis.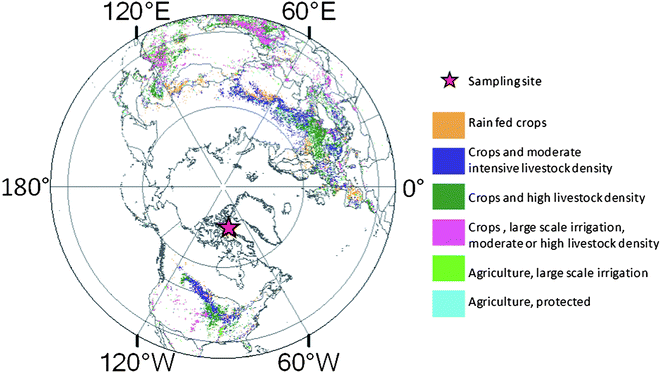 | ||
| Fig. 1 Map showing the sampling site on the Devon Island Ice Cap and the areas with agriculture activities (data from digital map of land use systems of the world by Food and Agriculture Organization, http://www.fao.org/geonetwork/srv/en/main.home). | ||
Sample preparation and analyses
Analytical methods and QA/QC have been described elsewhere18,31 and further details are provided in the ESI.† In brief, individual snow/ice samples were melted in a clean laboratory at CCIW (positively pressurized, HEPA and carbon filtered air) and the meltwater (8.5–12 L per sample) was extracted using XAD-2 resin columns.The columns were sequentially extracted with methanol (CH3OH) and dichloromethane (CH2Cl2) and the combined extracts were washed with 3% NaCl and dried on anhydrous Na2SO4. The extract was concentrated to a small volume under vacuum in a rotary evaporator and added to a small column of 10% H2O-deactivated silica-gel and eluted with 10% CH3OH–CH2Cl2. Acetone–isooctane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added and the sample was concentrated to a final volume of 0.2 mL. All CUPs were analyzed using GC-NCI-MS (low resolution MS). A 1 μL aliquot of each extract was injected into a GC (pulsed splitless injection at 250 °C) onto a 30 m × 0.25 mm diameter capillary column (5% diphenyl-/95% dimethylsiloxane liquid phase) (0.25 mm film thickness) at an initial GC oven temperature of 80 °C. 13C-mirex was added to each XAD column prior to elution as a method recovery standard. Mirex recoveries (N = 14 samples) averaged (± standard deviation, SD) 90 ± 12% and no recovery correction was made. Procedural blanks (N = 4) consisting of XAD and all reagents were analyzed with every 4 snow samples. XAD resin blanks (N = 4) consisting of the CH3OH–CH2Cl2 elution were also analyzed. Method detection limits (MDL) were calculated from the combined blank results (3 × SD, N = 8) or where blanks were non-detectable, using the instrument detection limit (IDL). All results were blank corrected using the average blank (N = 8). The full list of analytes, as well as MDLs and IDLs, is given in the ESI, Table S1.†
1) was added and the sample was concentrated to a final volume of 0.2 mL. All CUPs were analyzed using GC-NCI-MS (low resolution MS). A 1 μL aliquot of each extract was injected into a GC (pulsed splitless injection at 250 °C) onto a 30 m × 0.25 mm diameter capillary column (5% diphenyl-/95% dimethylsiloxane liquid phase) (0.25 mm film thickness) at an initial GC oven temperature of 80 °C. 13C-mirex was added to each XAD column prior to elution as a method recovery standard. Mirex recoveries (N = 14 samples) averaged (± standard deviation, SD) 90 ± 12% and no recovery correction was made. Procedural blanks (N = 4) consisting of XAD and all reagents were analyzed with every 4 snow samples. XAD resin blanks (N = 4) consisting of the CH3OH–CH2Cl2 elution were also analyzed. Method detection limits (MDL) were calculated from the combined blank results (3 × SD, N = 8) or where blanks were non-detectable, using the instrument detection limit (IDL). All results were blank corrected using the average blank (N = 8). The full list of analytes, as well as MDLs and IDLs, is given in the ESI, Table S1.†
Snow pit dating
As described by Meyer et al.,18 the dating of the snow pit, i.e. assigning deposition years to snow layers, was based on the physical snow profile, annual net accumulation, and inorganic meltwater chemistry. Physical snow profiles include snow densities, as well as snow grain structures and ice layers observed and recorded while working in the pit (Fig. S1 and S2† of Meyer et al.18). Historical accumulation data of water equivalents and modeled high resolution mass balance estimates for the Devon Ice Cap were taken from Boon et al.37 and from Gardner and Sharp,38 respectively. A detailed description of the dating procedure, associated calculations, and uncertainties can be found in the ESI of ref. 18.Trajectory and GIS analysis
The origin of air masses arriving at the sampling site on the Devon Ice Cap was traced with five-day backward trajectories generated using the Trajectory Model by Environment Canada.39 Trajectories arriving at a height of 50, 100 and 200 m above the sampling site were calculated every 6 h from 1994 to 2008. Reflecting the period of agricultural pesticide application in the temperate zone of the Northern hemisphere, only the data from May to September (inclusive) of each year were used to generate a map of the density of trajectory points with the point density tool of ArcGIS 10.0 (cell size 25 km and circle radius 200 km). These maps, generated under the North_Pole_Lambert_Azimuthal_Equal_Area coordinate system, are often referred to as “airsheds”,40 and were used to assess whether interannual differences in air mass origin could explain the deposition record from the snow pit. Due to the lack of quantitative information on pesticide use in different agricultural regions, we analyzed the contribution of agriculture activities (pesticide use) semi-quantitatively by superimposing the airsheds with a map of agricultural land use.41 The total area of agricultural lands within 10 km of each of the 7.0 × 104 trajectory endpoints for each summer was calculated with ArcGIS 10.0 and used as a semi-quantitative metric (referred to as the agriculture contribution index or ACI hereafter) to assess the relationship between measured pesticides deposition in the Arctic and potential contribution from agricultural applications.Results and discussion
Concentrations and deposition fluxes of current use pesticides
The concentrations of selected CUPs in the snow pit samples from 2008 are presented in Fig. S1.† Concentrations (water equivalent) ranged from <0.2 to 2 pg L−1 for trifluralin, <0.8 to 26 pg L−1 for pentachloronitrobenzene (PCNB), <1.5 to 52 pg L−1 for chlorothalonil, <0.5 to 40 pg L−1 for metribuzin, 3 to 40 pg L−1 for dacthal, <1 to 30 pg L−1 for α-endosulfan, <0.3 to 11 pg L−1 for β-endosulfan, 1.5 to 16 pg L−1 for endosulfan sulfate, and <0.5 to 23 pg L−1 for quizalofop ethyl. Chlorpyrifos, dimethoate, ethafluralin, myclobutanil, malathion, pendamethalin, propiconazole, phosmet, phosalone, and tefluthrin were not detected in any of the samples (Table S1†).Dacthal levels (water equivalent) in Devon Ice Cap snow were comparable with concentrations of 10–70 pg L−1 measured in the water of Canadian Arctic lakes (>70°N) sampled from 1999–2003,42 but were lower than the peak concentration of 300 pg L−1 (corresponding to the deposition year of 1979–1986) reported for an ice core from the Austfonna Ice Cap, Svalbard, Norway.2 While endosulfan sulfate concentrations in the snow were comparable to the concentrations in Arctic lake water (<10 pg L−1), the peak concentrations in the snow samples corresponding to the deposition years of 1993–1996 and 2002–2004 were higher than in Arctic lake water.42
Based on the annual accumulation of water equivalents (kg cm−2 per year) inferred from the density and thickness of each snow segment, the concentrations were converted to net atmospheric deposition fluxes (Fig. 2). These fluxes in units of pg cm−2 per year ranged from <0.002 to 0.04 for trifluralin, <0.01 to 0.6 for PCNB, <0.01 to 1.0 for chlorothalonil, <0.005 to 1.1 for metribuzin, 0.04 to 1.0 for dacthal, <0.002 to 0.6 for α-endosulfan, <0.003 to 0.2 for β-endosulfan, <0.02 to 0.4 for endosulfan sulfate, and <0.005 to 0.6 for quizalofop ethyl.
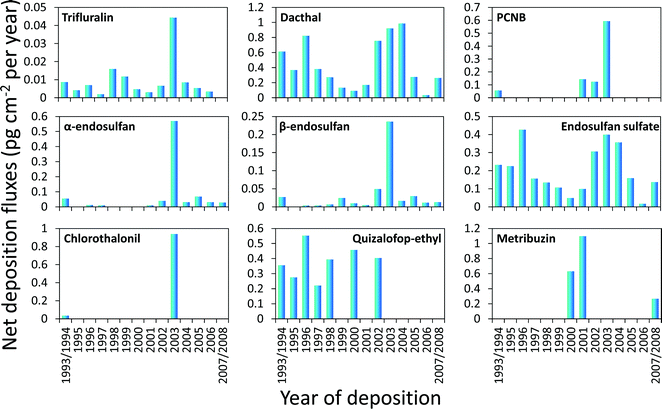 | ||
| Fig. 2 Annual net deposition fluxes of current use pesticides inferred from measured concentrations in snow segments from a snow pit dug on the Devon Island Ice Cap in 2008. | ||
The peak deposition flux of 1 pg cm−2 per year for dacthal was lower than the 1995–2005 deposition flux of 5 pg cm−2 per year inferred from ice core records at Holtedahlfonna, Svalbard, Norway.31 Fluxes of α-, β-endosulfans and endosulfan sulfate are lower than those at Holtedahlfonna by one order of magnitude.31 Fluxes of the endosulfan-group compounds were also lower than fluxes derived from concentrations in fresh snow from the Canadian Arctic,5 which could be caused by a significant fraction of previously deposited endosulfan undergoing re-volatilization due to strong katabatic winds occurring on the Devon Ice Cap.37
The herbicide trifluralin has a near-continuous profile in the snow core (Fig. 2), which is similar to the observation in the Holtedahlfonna ice core.31 However, the peak deposition flux of trifluralin at the Devon Ice Cap recorded for 2003 was 50 times lower than the flux measured at Holtedahlfonna.31 Fungicides PCNB and chlorothalonil were not continuously detected in the snow segments (Fig. 2). Quizalofop ethyl, a post-emergence herbicide for the selective control of annual and perennial grass weeds in broad-leaved crops, was detected in the snow deposited before the early 2000s at a level of ∼20 pg L−1 (Table S1†). In contrast, in the ice core from Holtedahlfonna, no quizalofop ethyl could be detected.31 The herbicide metribuzin was only detected in snow deposited in 2000–2001 and 2007–2008.
Comparing dacthal and endosulfan
By being detected in all the layers of the snow core (Fig. 2), dacthal and endosulfan sulfate were the most frequently detected CUPs. Dacthal is a chlorinated phthalate herbicide primarily used on vegetables. Registered for use only in the UK, Canada, USA, New Zealand and Australia,31 the dominant user is the USA with 270 ton per year.43 Use in Canada is a mere ∼1% of that in the USA.44 Endosulfan sulfate is the degradation product of endosulfan, an insecticide that is more widely produced and used around the globe. In the early 2000s, its global production was estimated to exceed 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ton per year.45
000 ton per year.45
Despite its much lower production volume and more limited geographical use area,43,45 dacthal concentrations were higher than those of the sum of endosulfan sulfate and its parent compounds (α- and β-endosulfan) in all snow samples. The only exception is the sample corresponding to the year 2003, in which both endosulfan isomers had levels about one order of magnitude higher than in other years. Generally higher concentrations of dacthal compared to the endosulfans are consistent with measurements in Arctic lake water, but contrast with what has been observed in the water of remote lakes at lower latitude (40–50°N).42 These observations provide empirical evidence for earlier model predictions46 that dacthal has a higher potential for long range atmospheric transport than the endosulfans. The efficiency of atmospheric deposition of dacthal and endosulfan sulfate should be similar because their physicochemical properties are similar.5,46 The higher potential of dacthal for long range transport can thus be attributed to a predicted atmospheric half-life (860 h) that is much longer than that of endosulfan (50 h) and endosulfan sulfate (50 h).31
Endosulfan composition
The composition of the endosulfans in the snow samples is shown in Fig. 3. Excluding the samples corresponding to deposition years 1997–1999, in which α-endosulfan was not detected, the average ratio between α- and β-endosulfan was 2.0 ± 0.7. This ratio is at the lower end of that reported for technical endosulfan (2 to 2.3),4,5 and the ratios of 2.6 to 3.4 were recorded in ice core segments from Holtedahlfonna, Svalbard, Norway.31 Weber et al.5 suggested that the difference in the α- and β-isomer ratio can reflect differences in the technical formulations of endosulfan, different degradation rates and sorption/partitioning properties of the two isomers, and possibly β- to α-isomer conversion. In the sample corresponding to deposition years 2001 and 2002, β-endosulfan was more abundant than α-endosulfan even though β-endosulfan is generally believed to degrade faster in the atmosphere.47 Similarly, a higher relative abundance of β-endosulfan was observed in lake water from mountains in Costa Rica.48 It can be inferred from such observations that other factors and processes, which remain to be identified, could have contributed to faster depletion of the α-isomer.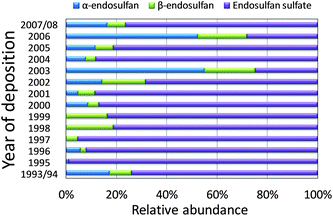 | ||
| Fig. 3 Relative abundance of α- and β-endosulfan and endosulfan sulfate in snow segments from a snow pit dug on the Devon Island Ice Cap in 2008. | ||
In the environment, endosulfan is subject to biotic and abiotic degradation that results in the formation of endosulfan sulfate. Therefore, the relative abundance of endosulfan sulfate serves as an indicator for the level of environmental degradation of the technical product. Except for the samples corresponding to years 2003 and 2006, endosulfan sulfate was more abundant than its parent compounds. This is in contrast to the pattern in the ice core from Holtedahlfonna, Svalbard, where endosulfan sulfate was less abundant than endosulfan. Because no trend in the relative abundance of endosulfan sulfate with time was observed, we infer that firn records the endosulfan composition at the time of deposition, i.e. no transformation has occurred after deposition. The higher abundance of the parent compounds in 2003 and 2006 was probably due to deposition of recently used endosulfan. Judging from the interannual variations of the airsheds (Fig. 4), these two years are quite unusual in that some air masses originated from Western Russia. Hermanson et al.2 also argued that deposition of some pesticides to ice caps in Svalbard could be accounted for by event-based long range atmospheric transport from agricultural areas in northern Eurasia. Possibly, the elevated deposition of trifluralin, PCNB and chlorothalonil in 2003 can also be related to the occurrence of air mass origin from Western Russia.
 | ||
| Fig. 4 Airsheds of the Devon Island Ice Cap sampling site during selected summer periods obtained by plotting the density of air mass backward trajectory endpoints (hot color represents high density; cold color represents low density). Stronger influence from Western Russia was observed in 2003 and 2006. | ||
Temporal variability of dacthal and endosulfan deposition
The temporal profiles of dacthal and endosulfan sulfate were quite similar: concentrations and deposition fluxes showed no significant trend from the early 1990s to the late 2000s, showed relatively large year to year variability and peaked around 1993–1996 and 2002–2004 (Fig. 2). Trifluralin, PCNB and chlorothalonil also had higher concentrations in the sample corresponding to the 2003 deposition year (Fig. 2). These years of maximum CUP deposition were different from those for the PBDEs, which occurred in 1999 and 2007/08.18 A different deposition history may reflect different source areas for the two groups of compounds: pesticides predominantly originate from agricultural lands while flame retardants predominantly originate from populated areas.The annual variation of BDE-209 deposition was explained by the fraction of trajectory endpoints originating from densely populated North America. A similar approach was adopted to assess whether air mass origin over agricultural lands, where the majority of CUPs presumably have been used, can explain the variations in the CUP deposition fluxes. Fig. 5 displays the annual variations of the agriculture contribution index (ACI), a semi-quantitative indicator for the level of agricultural influence on the air masses arriving at the sampling site, which is based on the airsheds assembled from the backward trajectories (Fig. S2†). Air mass origin and therefore also ACI vary from year to year. In 1994 and 1999, the 5-day trajectories reached further into temperate latitudes and thus both the fraction of trajectory endpoints passing densely populated area in North America, which was used as an indicator for the contribution of PBDE sources in a previous study,18 and the ACI are higher in these years. Efforts to relate the annual variations in air mass origin with the variations in measured CUP concentrations were not successful. For example, most of the CUPs had peak concentrations in the layer corresponding to the year 2003 but the ACI for 2003 was one of the lowest among the years. This is likely because the ACI relies on generic classifications of agricultural lands rather than geographically explicit data on CUP use. Also, while we considered only air mass origin during summer, pesticide application is sometimes confined to much shorter time periods.
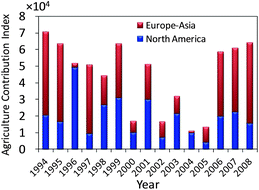 | ||
| Fig. 5 Agriculture contribution index (ACI) for air masses arriving at the sampling site at the Devon Ice Cap. ACI is defined as the total area of agricultural land within 10 km of each of the 7.5 × 104 trajectory endpoints based on 6-hour interval air mass backward trajectories from May to September (inclusive) of each year. The contributions of endpoints located in North America and Eurasia to ACI are distinguished. | ||
Geographically explicit annual use data for dacthal and endosulfan in the United States from 1992–2009 are available from the Pesticide National Synthesis Project of the U.S. Geological Survey (Fig. S3 and S4†).49 Although pesticide use in regions other than the US can contribute to the CUP deposition in the Arctic, the peak in the deposition of dacthal and endosulfan to the Devon Ice Cap in 1996 and in 2002–2004 can partially be explained by the annual dacthal and endosulfan use maps in the US together with the airshed maps (Fig. S2†). In 1996, a higher portion of the trajectory endpoints overlapped with the agricultural land stretching from the southwest of the Great Lakes to the Canadian Prairies; dacthal and endosulfan happened to be used more in this area in 1996 than in 1995 and 1997. In 2002–2004, the annual use of dacthal in the same region was higher than in 2001 and after 2005, which could have caused the elevated dacthal deposition at Devon during these years.
Inventory estimates of CUPs in the ice caps of the Canadian Arctic Archipelago
Seasonal glacial melt was shown to be a major source of pesticides to a subalpine lake.22 Similarly, global warming may enhance the release of chemicals from the Arctic cryosphere.50 Therefore, knowledge of the stock of contaminants in the Arctic cryosphere is required when seeking to assess the impact of future climate change on the release and impact of Arctic contaminants.51,52 The amount of a chemical stored in an Arctic ice cap reflects its historical uses in source regions and its potential to undergo long range transport and deposition and therefore serves as an empirical indicator for assessing the environmental impact of the chemical. The inventory of CUPs in Canadian Arctic glaciers due to deposition between 1993 and 2008 was estimated by assuming that the amount in the snow layers measured in this study is applicable to the entire area of 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2 covered by ice caps in the Arctic Archipelago,53 even though we are aware that extrapolating measured concentrations in one location to a larger area is fraught with large uncertainties because of the spatial variation in the chemical deposition fluxes. Dacthal had the highest stored amount of 9 kg followed by endosulfan sulfate, quizalofop-ethyl and metribuzin, with 4, 4 and 3 kg, respectively. The stocks for the rest of the CUPs were below 2 kg (Fig. S5b†). While these inventories are very unlikely to notably impact concentrations in Arctic Ocean water if climate change were to accelerate ice cap melting, they could impact lakes receiving glacial meltwater. Examples of such lakes include Bear Lake, which receives meltwater from the Devon Ice Cap,54 Lake Hazen which receives meltwater from ice caps on the northern Ellesmere Island,55 and Nettilling Lake which receives meltwater from the Penny Ice Cap.56
000 km2 covered by ice caps in the Arctic Archipelago,53 even though we are aware that extrapolating measured concentrations in one location to a larger area is fraught with large uncertainties because of the spatial variation in the chemical deposition fluxes. Dacthal had the highest stored amount of 9 kg followed by endosulfan sulfate, quizalofop-ethyl and metribuzin, with 4, 4 and 3 kg, respectively. The stocks for the rest of the CUPs were below 2 kg (Fig. S5b†). While these inventories are very unlikely to notably impact concentrations in Arctic Ocean water if climate change were to accelerate ice cap melting, they could impact lakes receiving glacial meltwater. Examples of such lakes include Bear Lake, which receives meltwater from the Devon Ice Cap,54 Lake Hazen which receives meltwater from ice caps on the northern Ellesmere Island,55 and Nettilling Lake which receives meltwater from the Penny Ice Cap.56
Explaining the high interannual variability in deposition
The concentrations of the CUPs measured in the snow samples vary widely between different annual layers, sometimes by as much as one order of magnitude from one year to the next. Similar variations were observed for PBDEs analyzed in the same samples. Pesticides analyzed in ice cores from ice caps on Svalbard, Norway show similarly high interannual variability.2,18,31 Because it is unlikely that emission rates of these substances experience such large interannual variations (e.g. the total uses of dacthal and endosulfan in North Dakota, Minnesota and Wisconsin were higher in 1996 than in 1994 and 1995 by only about a factor of two49), this variability suggests that factors controlling the transport and deposition of CUPs to the ice cap are highly variable from year to year. For a CUP to be transported and deposited to the sampling site at the Devon Island Ice Cap (∼1500 m above sea level) the following has to happen: an air mass originating in an agricultural source region during a time of pesticide application has to be transported to relatively high elevations (above the height of the ice cap) within the Arctic during a relatively short amount of time (generally a few days, to limit the extent of atmospheric degradation) and without encountering significant precipitation along the way, but then should experience snow fall on the ice cap for efficient deposition to take place. Because all these conditions have to be met for efficient transport and deposition of CUPs to the ice cap to occur, it is conceivable that they prevail very rarely. If transport and deposition of CUPs were to occur continuously or at least with a reasonable frequency during the course of a year, we would expect a less variable deposition profile. Therefore, the high interannual variability of the CUP deposition profiles observed in this study suggests that a few episodic events contribute the majority of the loadings to the Arctic surface.Measurements of pesticides in precipitation at reasonably high temporal resolution generally do indeed show a highly episodic character.57–59 In source regions, the occurrence of high concentrations in rain depends on the timing of pesticide application and precipitation. As a result, even over extended time periods, deposition fluxes can be dominated by a few events. For example, concentrations of HCHs measured in precipitation of southern Norway at a weekly temporal resolution varied by as much as two orders of magnitude.60 A few episodic events with high concentrations contributed the majority of the total wet HCH deposition over the course of a whole year.60 If this is the case at sampling sites in close proximity to regions with pesticide application, episodicity of pesticide deposition will only be more pronounced in remote Arctic regions. Even though they may be influenced by many more source regions, very few air masses that picked up pesticides during an application event will find their way to the Arctic without encountering precipitation along the way.
This high episodicity of pesticide deposition on Arctic ice caps also questions their suitability as archives recording temporal trends in the transport and deposition of contaminants to the Arctic. The very high interannual variability compromises their use as reliable indicators of pesticide use on a hemispheric scale, at least at the annual resolution. Drilled snow cores, that are deeper than the pits investigated here and thus cover much longer time periods, may be suitable for determining long term trends in pesticide usage over the decadal time scale. The primary value of relatively shallow snow pits lies in providing proof of the atmospheric transport and deposition of a contaminant in the Arctic.
Acknowledgements
We thank Dan Walsh (Environment Canada, Burlington ON) for organizing and conducting the ice cap sampling. We thank Alex Gardner for providing the Devon Ice Cap mass balance dataset. Funding (to DCGM) was provided by the Northern Contaminants Program, Aboriginal Affairs and Northern Development Canada and by Environment Canada's Chemicals Management Plan. XZ acknowledges the support by an Ontario Postdoctoral Fellowship.References
- L. Hoferkamp, M. H. Hermanson and D. C. G. Muir, Sci. Total Environ., 2010, 408, 2985–2994 CrossRef CAS PubMed.
- M. H. Hermanson, E. Isaksson, C. Teixeira, D. C. G. Muir, K. M. Compher, Y. Li, M. Igarashi and K. Kamiyama, Environ. Sci. Technol., 2005, 39, 8163–8169 CrossRef CAS PubMed.
- C. P. Rice and S. M. Chernyak, Chemosphere, 1997, 35, 867–878 CrossRef CAS.
- J. Weber, C. J. Halsall, D. C. G. Muir, C. Teixeira, D. A. Burniston, W. M. J. Strachan, H. Hung, N. Mackay, D. Arnold and H. Kylin, Environ. Sci. Technol., 2006, 40, 7570–7576 CrossRef CAS PubMed.
- J. Weber, C. J. Halsall, D. Muir, C. Teixeira, J. Small, K. Solomon, M. Hermanson, H. Hung and T. Bidleman, Sci. Total Environ., 2010, 408, 2966–2984 CrossRef CAS PubMed.
- A. A. Boyd-Boland, S. Magdic and J. B. Pawliszyn, Analyst, 1996, 121, 929–937 RSC.
- M. Oehme, Ambio, 1991, 20, 293–297 Search PubMed.
- R. M. Hoff and K. W. Chan, Chemosphere, 1986, 15, 449–452 CrossRef CAS.
- G. W. Patton, D. A. Hinckley, M. D. Wallai, T. F. Bidleman and B. T. Hargrave, Tellus, Ser. B, 1989, 41, 243–255 CrossRef.
- A. T. Fisk, C. A. de Wit, M. Wayland, Z. Z. Kuzyk, N. Burgess, R. Robert, B. Braune, R. Norstrom, S. P. Blum, C. Sandau, E. Lie, H. J. S. Larsen, J. U. Skaare and D. C. G. Muir, Sci. Total Environ., 2005, 351, 57–93 CrossRef PubMed.
- W. E. Cotham and T. F. Bidleman, Chemosphere, 1991, 22, 165–188 CrossRef CAS.
- Y. D. Lei and F. Wania, Atmos. Environ., 2004, 38, 3557–3571 CrossRef CAS.
- T. Meyer, Y. D. Lei, I. Muradi and F. Wania, Environ. Sci. Technol., 2009, 43, 657–662 CrossRef CAS PubMed.
- T. Meyer, Y. D. Lei, I. Muradi and F. Wania, Environ. Sci. Technol., 2009, 43, 663–668 CrossRef CAS PubMed.
- T. Meyer and F. Wania, Water Res., 2011, 45, 3627–3637 CrossRef CAS PubMed.
- J. R. Garbarino, E. Snyder-Conn, T. J. Leiker and G. L. Hoffman, Water, Air, Soil Pollut., 2002, 139, 183–214 CrossRef CAS.
- B. M. J. Herbert, S. Villa and C. Halsall, Ecotoxicol. Environ. Saf., 2006, 63, 3–16 CrossRef CAS PubMed.
- T. Meyer, D. C. Muir, C. Teixeira, X. Wang, T. Young and F. Wania, Environ. Sci. Technol., 2012, 46, 826–833 CrossRef CAS PubMed.
- T. J. Peterle, Nature, 1969, 224, 620 CrossRef CAS PubMed.
- D. A. Peel, Nature, 1975, 254, 324–325 CrossRef CAS.
- D. J. Gregor and W. D. Gummer, Environ. Sci. Technol., 1989, 23, 561–565 CrossRef CAS.
- J. M. Blais, D. W. Schindler, D. C. G. Muir, L. E. Kimpe, D. B. Donald and B. Rosenberg, Nature, 1998, 395, 585–588 CrossRef CAS.
- G. Carrera, P. Fernández, R. M. Vilanova and J. O. Grimalt, Atmos. Environ., 2001, 35, 245–254 CrossRef CAS.
- K. J. Hageman, S. L. Simonich, D. H. Campbell, G. R. Wilson and D. H. Landers, Environ. Sci. Technol., 2006, 40, 3174–3180 CrossRef CAS PubMed.
- A. J. Peters, D. J. Gregor, C. F. Teixeira, N. P. Jones and C. Spencer, Sci. Total Environ., 1995, 160–161, 167–179 CrossRef.
- D. J. Gregor, A. J. Peters, C. Teixeira, N. Jones and C. Spencer, Sci. Total Environ., 1995, 160–161, 117–126 CrossRef.
- J. L. Jaffrezo, M. P. Clain and P. Masclet, Atmos. Environ., 1994, 28, 1139–1145 CrossRef CAS.
- P. Masclet, V. Hoyau, J. L. Jaffrezo and H. Cachier, Atmos. Environ., 2000, 34, 3195–3207 CrossRef CAS.
- D. B. Donald, J. Syrgiannis, R. W. Crosley, G. Holdsworth, D. C. G. Muir, B. Rosenberg, A. Sole and D. W. Schindler, Environ. Sci. Technol., 1999, 33, 1794–1798 CrossRef CAS.
- E. Isaksson, M. Hermanson, H. C. Sheila, M. Igarashi, K. Kamiyama, J. Moore, H. Motoyama, D. Muir, V. Pohjola, R. Vaikmae, R. S. W. van de Wal and O. Watanabe, Phys. Chem. Earth, 2003, 28, 1217–1228 CrossRef.
- R. M. Ruggirello, M. H. Hermanson, E. Isaksson, C. Teixeira, S. Forsstrom, D. C. G. Muir, V. Pohjola, R. van de Wal and H. A. J. Meijer, J. Geophys. Res.: Atmos., 2010, 115, D18308 CrossRef.
- P. Kukučka, G. Lammel, A. Dvorská, J. Klánova, A. Möller and E. Fries, Environ. Chem., 2010, 7, 504–513 CrossRef.
- M. Krachler, J. Zheng, R. Koerner, C. Zdanowicz, D. Fisher and W. Shotyk, J. Environ. Monit., 2005, 7, 1169–1176 RSC.
- C. J. Young, V. I. Furdui, J. Franklin, R. M. Koerner, D. C. G. Muir and S. A. Mabury, Environ. Sci. Technol., 2007, 41, 3455–3461 CrossRef CAS PubMed.
- D. A. Burniston, W. J. M. Strachan, J. T. Hoff and F. Wania, Environ. Sci. Technol., 2007, 41, 4932–4937 CrossRef CAS PubMed.
- B. M. J. Herbert, C. J. Halsall, S. Villa, K. C. Jones and R. Kallenborn, Environ. Sci. Technol., 2005, 39, 2998–3005 CrossRef CAS PubMed.
- S. Boon, D. O. Burgess, R. M. Koerner and M. J. Sharp, Arctic, 2010, 63, 13–29 CrossRef.
- A. S. Gardner and M. Sharp, Ann. Glaciol., 2009, 50, 80–86 CrossRef.
- R. D'Amours and P. Pagé, Atmospheric transport models for environmental emergencies, http://collaboration.cmc.ec.gc.ca/cmc/cmoi/product_guide/docs/lib/model-eco_urgences_e.pdf, accessed July 2013, 2001.
- J. N. Westgate and F. Wania, Environ. Sci. Technol., 2011, 45, 8850–8857 CrossRef CAS PubMed.
- Food and Agriculture Organization, Digital map of land use systems of the world, http://www.fao.org/geonetwork/srv/en/main.home, accessed May 2013.
- D. Muir, C. Teixeira, M. Alaee and M. Hermanson, in Persistent Organic Pollutants (POPs) in the European Atmosphere: An Updated Overview. European Commission, JRC/IES, ed. J. Castro-Jiménez, S. J. Eisenreich and I. Vives, 2007. pp. 88–95 Search PubMed.
- National Center for Food and Agricultural Policy, National Pesticide Use Database, http://www.ncfap.org/database/national.php, accessed June 2013.
- Environment Canada Pesticide Program Coordinating Committee, Pesticide utilization in Canada: a compilation of current sales and use data, Environment Canada, 2005 Search PubMed.
- Y. F. Li and R. W. Macdonald, Sci. Total Environ., 2005, 342, 87–106 CrossRef CAS PubMed.
- D. C. G. Muir, C. Teixeira and F. Wania, Environ. Toxicol. Chem., 2004, 23, 2421–2432 CrossRef CAS PubMed.
- G. Zhang, P. Chakraborty, J. Li, P. Sampathkumar, T. Balasubramanian, K. Kathiresan, S. Takahashi, A. Subramanian, S. Tanabe and K. C. Jones, Environ. Sci. Technol., 2008, 42, 8218–8223 CrossRef CAS PubMed.
- C. Shunthirasingham, T. Gouin, Y. D. Lei, C. Ruepert, L. E. Castillo and F. Wania, Environ. Toxicol. Chem., 2011, 30, 2709–2717 CrossRef CAS PubMed.
- G. P. Thelin and W. W. Stone, Estimated annual agricultural pesticide use for counties of the conterminous United States, 1992–2009: U.S. Geological Survey Scientific Investigations Report 2013-5009, 2013 Search PubMed.
- P. D. Noyes, M. K. McElwee, H. D. Miller, B. W. Clark, L. A. Van Tiem, K. C. Walcott, K. N. Erwin and E. D. Levin, Environ. Int., 2009, 35, 971–986 CrossRef CAS PubMed.
- J. M. Armitage, C. L. Quinn and F. Wania, J. Environ. Monit., 2011, 13, 1532–1546 RSC.
- T. Meyer and F. Wania, Atmos. Environ., 2007, 41, 2757–2767 CrossRef CAS.
- C. S. Ommanney, U.S.Geological Survey Professional Paper(1386-J), 2002, pp. 83–110 Search PubMed.
- S. F. Lamoureux and R. Gilbert, Quat. Res., 2004, 61, 134–147 CrossRef.
- I. R. Smith, Can. J. Earth Sci., 1999, 36, 1547–1565 CrossRef.
- K. Gajewski and D. Atkinson, Environ. Rev., 2003, 11, 69–102 CrossRef.
- D. F. K. Rawn, T. H. J. Halldorson, B. D. Lawson and D. C. G. Muir, J. Environ. Qual., 1999, 28, 898–906 CrossRef CAS.
- A. Goel, L. L. McConnell and A. Torrents, J. Agric. Food Chem., 2005, 53, 7915–7924 CrossRef CAS PubMed.
- A. Scheyer, S. Morville, P. Mirabel and M. Millet, Atmos. Environ., 2007, 41, 7241–7252 CrossRef CAS.
- F. Wania and J. E. Haugen, Environ. Pollut., 1999, 105, 381–386 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figures showing concentrations in the snow segments in snow water equivalent, airsheds of the Devon Island Ice Cap sampling site for all investigated deposition years, and maps for the use of dacthal and endosulfan in the USA during the investigated deposition years. See DOI: 10.1039/c3em00433c |
| This journal is © The Royal Society of Chemistry 2013 |
