 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering
Ahmed
Al-Kattan
ab,
Adrian
Wichser
a,
Roger
Vonbank
c,
Samuel
Brunner
c,
Andrea
Ulrich
a,
Stefano
Zuin
d and
Bernd
Nowack
*b
aEmpa - Swiss Federal Laboratories for Material Science and Technology, Laboratory for Analytical Chemistry, Überlandstrasse 129, 8600 Dübendorf, Switzerland
bEmpa - Swiss Federal Laboratories for Material Science and Technology, Technology and Society Laboratory, Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland. E-mail: nowack@empa.ch; Fax: +41 (0)712747862; Tel: +41 (0)712747692
cEmpa - Swiss Federal Laboratories for Material Science and Technology, Laboratory for Building Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland
dVenice Research Consortium, Via della Libertà 12, c/o VEGA park, 30175 Venice, Italy
First published on 30th August 2013
Abstract
The release of nanomaterials from products and applications that are used by industry and consumers has only been studied to a very limited extent. The amount and the characteristics of the released particles determine the potential environmental exposure. In this work we investigated the release of Ti from paints containing pigment-TiO2 and nano-TiO2. Panels covered with paint with and without nano-TiO2 were exposed to simulated weathering by sunlight and rain in climate chambers. The same paints were also studied in small-scale leaching tests to elucidate the influence of various parameters on the release such as composition of water, type of support and UV-light. Under all conditions we only observed a very low release close to background values, less than 1.5 μg l−1 in the climate chamber over 113 irrigations per drying cycle and between 0.5 and 14 μg l−1 in the leaching tests, with the highest concentrations observed after prolonged UV-exposure. The actual release of Ti over the 113 weathering cycles was only 0.007% of the total Ti, indicating that TiO2 was strongly bound in the paint. Extraction of UV-exposed and then milled paint resulted in about 100-times larger release of Ti from the nano-TiO2 containing paint whereas the paint with only pigment-TiO2 did not show this increase. This indicated that the release of Ti from the paints is an effect of the addition of nano-TiO2, either by photocatalytic degradation of the organic paint matrix (observed by electron microscopic imaging of the paint surface) or by direct release of nano-TiO2. Our work suggests that paints containing nano-TiO2 may release only very limited amounts of materials into the environment, at least over the time-scales investigated in this work.
Environmental impactPaints containing engineered nanomaterials are already on the market but little is known about the potential for release. In this paper we are quantifying and characterizing (nano)materials released from the nano-paint during weathering into water. Release is a prerequisite for exposure and the characterization of release is therefore a crucial component of the environmental risk assessment of nanomaterials. |
Introduction
In the last few years a lot of knowledge has been acquired on the behavior and effects of engineered nanomaterials (ENM) in the environment. Many studies have investigated processes such as agglomeration, dissolution, removal during wastewater treatment or transformation reactions.1–4 Also the possible effects of ENM on environmental organisms have received a lot of attention and a vast body of research has been performed so far.5,6 However, in contrast to all the fate and effect studies, very little is actually known about the release of ENM into the environment.7 Currently, techniques are not available for most ENM to specifically monitor their release and quantify their concentrations in the environment at trace levels8,9 and thus reports on release and environmental concentrations are very rare.In addition to knowledge about the amounts of ENM released into the environment, it is equally important to investigate in what form they are released.10 The results from the available studies show that a large fraction of the released ENM is present in the matrix-bound form and that only a small fraction is released as single nanoparticles. Almost all fate and effect studies so far have been performed with pristine ENM – the materials that are synthesized by scientists or companies. However, these ENM undergo transformation and aging reaction during use and release.10 The materials that actually reach the environment may be completely different from the materials originally produced by industry. We therefore urgently need information on the amount and characteristics of the materials that are actually released under real-world conditions.
The use of ENM in paints and coatings is an important application area.11,12 The ENM are used as biocides and additives for protection against microbial, physical and chemical deterioration.13 It has been shown by Kaegi et al. that TiO2 nanoparticles are released from buildings into the aquatic environment.14 These authors traced particles emitted from exterior facade paints into surface waters. By combining results from microscopic investigations with bulk chemical analysis, the authors were able identify and quantify the released nano-TiO2 particles. It has to be noted, however, that the buildings investigated in this work did not contain any nano-paint, the released nanoparticles originated from normal paint with pigment-TiO2. It has been shown that up to 36% of the particle numbers in a pigment-TiO2 sample was smaller than 100 nm,15 indicating that for a source appointment of nano-TiO2 not only the presence of nanoparticles is sufficient to prove release of ENM, but also their characterization is needed.
A second study investigated the release of metallic silver nanoparticles (Ag-NPs) from a paint used for outdoor applications.16 A facade panel mounted on a model house was exposed to ambient weather conditions for a period of one year. A strong leaching of the Ag-NPs was observed during the initial runoff events with a maximum concentration of 145 μg Ag per l. After a period of one year, more than 30% of the Ag-NPs were released into the environment. The particles were mostly <15 nm and were released as composite colloids attached to the organic binder of the paint. Microscopic results indicated that the Ag-NPs are likely transformed into considerably less toxic forms such as Ag2S.17
Whereas these investigations targeted the release under real-world or natural conditions, it is also possible to apply laboratory based leaching techniques to study release under more controlled conditions. An experimental protocol to simulate the aging of ENM-containing coatings under a water flow was proposed and applied to several nano-TiO2-containing coatings.18 TiO2 concentrations of up to 31 μg l−1 were detected in the immersion water, indicating some release of TiO2 nanoparticles. The effects of ENM in paint after abrasion by sanding of the paint has also been studied,19 however, this process is relevant for human exposure but not primarily for environmental release. The abraded particles were mainly in the μm range and the smallest particles (50 nm) originated from the sander.20 The presence of nanoparticles did not change the size distribution significantly compared to a nano-free reference paint.
The understanding of the magnitude and the form of the released materials is crucial for assessing their ecological and human health effects. It is well known that materials contained in paint can have very deleterious consequences on human health as for example in the case of leaded paint.21 The pathway from paint falling off walls to soil and by ingestion of soil to humans, especially children, has been shown to be one of the major sources of lead uptake with severe consequences on human health in cities.22 It is therefore essential to carefully study the behavior of the nano-TiO2-containing paint over the whole life-cycle.23
The very few previous studies about release from nano-paints pointed out that a release of nanoparticles is possible but the factors that determine the magnitude and form of release are not yet understood. The aim of this work was therefore to quantify release from paints with and without ENM under controlled conditions, identify the released materials and investigate the factors that influence release. The work was performed with the most important ENM used in paints, nano-TiO2, in a paint matrix containing pigment-TiO2 and with a reference paint with only pigment-TiO2.
Materials and methods
Samples
Paints with and without nanoparticles were provided by industrial project partners: paint A1 contained nano-TiO2 and pigment-TiO2 and paint A2 contained only pigment-TiO2. The composition of the paints is given in Table 1. The two TiO2 forms added to the paint were nano-TiO2 (Hombikat UV 100 WP, an aqueous dispersion of 50% nano-TiO2 (anatase) stabilized with polyacrylate, information from manufacturer) and pigment-TiO2 (RC823 by Cinkarna). Fig. 1 shows TEM images of the two TiO2 forms before adding to the paint. The pigment-TiO2 was composed of elongated particles in the range from 100 to 300 nm and the nano-TiO2 was in the range from 20–80 nm.| Label | A1 with pigment-TiO2 and nano-TiO2 | A2 with pigment-TiO2 |
|---|---|---|
| TiO2 rutile pigment | 135.8 | 165.8 |
| Nano-TiO2 anatase slurry (50%) | 60 | 0 |
| Water | 132.7 | 162.7 |
| Soya lecithin | 3.1 | 3.1 |
| NaOH solution 10% | 3.1 | 3.1 |
| Talkum filler | 65.8 | 65.8 |
| Grinded calcium carbonate filler | 317.5 | 317.5 |
| Styrene-acrylic copolymer dispersion (50% solids) | 146.2 | 146.2 |
| Silicone defoamer | 109.7 | 109.7 |
| Potassium siliconate | 10.4 | 10.4 |
| Coalescing agent | 8.4 | 8.4 |
| Biocide acticide MBS (MIT/BIT) | 3.1 | 3.1 |
| Polyurethane thickener (solids content 25%) | 4.2 | 4.2 |
| Sum | 1000 | 1000 |
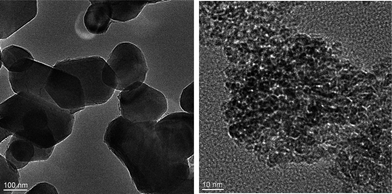 | ||
| Fig. 1 TEM images of pigment TiO2 (left) and nano-TiO2 (right) used in the paints. | ||
The paints were applied onto large panels of fiber cement (195 × 75 cm) at a concentration of 350 g m−2 wet paint and used in the weathering chamber experiments. Small panels of 10 × 10 cm were prepared for laboratory experiments. For these experiments paints A1 and A2 were applied to fiber cement and rendered on EPS (expanded polystyrol) at a concentration of 238 ± 35 g m−2. One face of each panel was painted with a first paint layer, dried for 48 hours before a second layer was applied. The panels were dried for 7 days before use. The unpainted sides of the panel were covered with wax to prevent any contribution of the matrix.
The aged paint was prepared as follows: A1 and A2 paints were applied on plastic (polyvinyl chloride; PVC) sheets by a manual film applicator. The wet thickness paint film applied was 200 μm for both paint samples. After drying for 24 h under indoor conditions, both paints were scraped off from the PVC panels by a plastic spatula and collected. The removed paint was milled in a planetary mill (Fritsch Planetary Mill Pulverisette 4 classic line). The milling was carried out in an yttria stabilized zirconia (YSZ) vial (i.e. container) and milling balls. The ball![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) powder ratio (charge ratio, CR) used was 2
powder ratio (charge ratio, CR) used was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This low charge ratio and the use of zirconia grinding media produces milled powders with little contamination from milling balls.24 In addition, milling media (i.e. vials and balls) were initially conditioned by using 70% EtOH
1. This low charge ratio and the use of zirconia grinding media produces milled powders with little contamination from milling balls.24 In addition, milling media (i.e. vials and balls) were initially conditioned by using 70% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (v/v) solution (milliQ water). The milling was performed for 40 minutes with 320 rpm rotor speed. The milled powders were then exposed to UVA light in an accelerated weathering machine. A fluorescent UV-A lamp (PHILIPS TL20W/09N) was used for irradiation, and the machine was designed to allow UV irradiation among paint powders by continuously shaking at low intensity for 500 hours. Philips 20W/09 lamps emit radiation over the whole UVA part of the spectrum (315–400 nm), with a power of 20 W. The duration of UV exposure was based on an existing standard (i.e., ISO 11507:2007) and the weathering machine was designed to reproduce damaging effects of sunlight.
water (v/v) solution (milliQ water). The milling was performed for 40 minutes with 320 rpm rotor speed. The milled powders were then exposed to UVA light in an accelerated weathering machine. A fluorescent UV-A lamp (PHILIPS TL20W/09N) was used for irradiation, and the machine was designed to allow UV irradiation among paint powders by continuously shaking at low intensity for 500 hours. Philips 20W/09 lamps emit radiation over the whole UVA part of the spectrum (315–400 nm), with a power of 20 W. The duration of UV exposure was based on an existing standard (i.e., ISO 11507:2007) and the weathering machine was designed to reproduce damaging effects of sunlight.
Weathering experiment
The weathering tests were performed with the large panels coated with the two paints in a weathering chamber. The artificial weathering consisted of 113 cycles of 6 hours each (3 hours of UV light, 0.5 h of irrigation and 2.5 h of drying). UV-A light with 315–400 nm (13.6 W (m−2 h−1)) and UV-B with 280–315 nm (3 W (m−2 h−1)) were used (Osram Ultral-Vitaux 300 W), representative of natural sunlight according to the producer OSRAM. The temperature during UV exposure was monitored and averaged 50 °C. The conditions were chosen based on the European norm for testing of facades.25 An average of 46 l of tap water (pH of 7.95) per cycle was dispersed via fine nozzles over the whole exposed wall panel during the irrigation stage. The runoff water was collected in plastic containers mounted at the bottom of the climate chamber. To quantify the Ti-contribution from irrigation water and the chamber, a control experiment with a polymethylmethacrylate (PMMA) panel instead of the painted fiber cement panel was performed for 28 cycles (chamber blank). Before the experiments the chambers were cleaned by flushing with tap water for 24 hours. At selected times, samples of the leachate water were collected and size fractionated as described below.Laboratory tests
Leaching experiments with small panels were performed under laboratory conditions in order to better understand the effects of parameters such as composition of medium, age of the paint, type of support, and effect of UV exposure on the release of Ti. These tests were performed with the 10 × 10 cm panels in small polypropylene containers of 17 cm × 17 cm × 6 cm. The panels were placed on two 1 cm high polypropylene sockets and 200 ml of the medium was added. The containers were placed on a horizontal shaker with a frequency of 32 min−1 for 24 hours. The leachate was then size fractionated as described below.Panels with paint A1 were exposed to the following media: deionized water, tap water and rain water (collected on the roof of the institute). The effect of the type of support was tested by using a panel of EPS coated with plaster and painted with A1. Paint A2 on the fiber cement was leached with deionized water only. A panel painted with A1 that was stored for 2 years under room temperature in the dark was leached with pure water. Additional leaching tests with panels A1, A1 aged and A2 that were placed for 3 weeks under a UVA Ultra Vitalux lamp (300 W) (70 cm of distance) were performed with deionized water. Panels of A1 and A1-aged were also exposed outdoors for 3 weeks to sunlight and leached with distilled water. The panels were placed on the support inclined at 45° and protected from rain by a transparent Perspex cover. All leaching experiments were performed in duplicate. Blank tests were performed with deionized water.
Extraction of aged paint powder
A suspension of 2 g l−1 of aged and powdered paints A1 and A2 was prepared in milli-Q water and stirred for 24 hours. The suspension was then kept for 24 hours without agitation to allow for sedimentation of large particles. Afterwards an aliquot of the supernatant was sampled and size-fractionated as described below.Size fractionation of leachates
All samples were size fractionated according to a sequential filtration procedure. 250 ml of raw materials (200 ml for laboratory tests) were first filtered through cellulose filters of 0.45 μm (Sartorius Stedim). An aliquot of the filtrate was filtered through a 0.1 μm polycarbonate/polyester membrane filter (Whatman). An aliquot of this solution was then filtered using an Amicon Ultra centrifugal filter (cut-off 30 kDa) for 10 minutes at 4000g. All filtrates were acidified using HNO3. The 0.45 μm filters of the climate chamber experiments were digested using KOH according to the method described by ref. 26 and analyzed for total Ti.Elemental analysis
Matrix elements (Ca, K, Mg, Na, Zn, Si, S and P) in the leachates were quantified by ICPOES (Vista Pro). Ti was quantified using ICPMS (Elan 6000). For calibration multielement standard solutions from Merck (Ca, K, Mg, Na and Zn) and single element standards from Alfa Aesar (Ti, Si, S and P) were used. As an internal standard Y was used for ICPOES and Rh for ICPMS. The detection limit for Ti was 0.1 μg l−1.Electron microscopy
The surface of the painted panels before and after weathering was characterized by scanning electron microscopy (SEM) on a Philips XL30-ESEM-FEG operated at 300 kV. Energy dispersive X-ray (EDX) analysis was used to identify TiO2 particles. Pieces of paint with a diameter of 1 cm were removed from the panels and coated with 5 nm of platinum before analysis.For leachate analysis, particles were deposited on TEM grids by centrifugation at 5000×g for 60 minutes. Transmission electron microscopy (TEM) was performed on Joel JEM-2200FS operated at an accelerated voltage of 200 kV, coupled with an energy dispersive X-ray (EDX) detector for elemental analysis.
Results
Climate chamber experiments
To assess the materials released from the panels, several elements typical for the paint matrix (Ca, Si, K, Mg, Na, P, S, Zn) as well as the potentially released nanoparticles of interest were measured by ICPOES and ICPMS. The concentrations were evaluated in the run-off solutions from chamber experiments filtered with 0.45 μm filters. The weathering chamber and irrigation water contribution (chamber blank) was subtracted and thus only the contribution of the paint was quantified. The irrigation water contained a background of Ca, Mg, K, Na, P, S and Ti of 65.4, 1.3, 10.3, 8.5, 0.04, 5.5 and 0.0014 mg l−1. The results for the release of elements typically present in the matrix composition of paints are shown in Fig. 2. For A1 and A2, Ca and S were found in a substantial amount in comparison to the other elements (K, Mg, P). In A1 no trend over time was observed and in A2 the Ca concentration was initially a bit higher than after 30 cycles.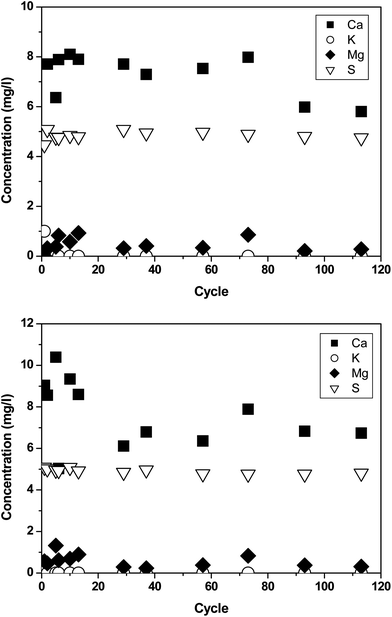 | ||
| Fig. 2 Release of Ca, Mg, S, and K from paints A1 (top) and A2 (bottom) in the weathering chamber. | ||
Fig. 3 shows the results for the Ti-concentrations in the leachate for the paints A1 (nano-TiO2 and pigment-TiO2) and A2 (pigment-TiO2). The Ti concentrations of A1 and A2 in the run-off waters were very low initially (about 1.5 μg l−1) and showed a decreasing trend over time. After 10 cycles the released concentrations stabilized at about 0.7 μg l−1. The paint containing nano-TiO2 did not release more Ti than the reference paint. The percentage of TiO2 particles leached from the paint can be calculated from the measured concentration and the recorded volume of the irrigation water. Of the 85 g of nano-TiO2 and pigment-TiO2 that were present on the panel, about 0.007% were detected in the drainage water. Panel A2 resulted in similar concentrations of Ti in the leachate (around 1 μg l−1), with a cumulative release of about 0.007% of TiO2 (this paint contained the same amount of TiO2 as A1). Thus, both the pigment and the nanoparticulate TiO2 seem to be well embedded in the paint matrix.
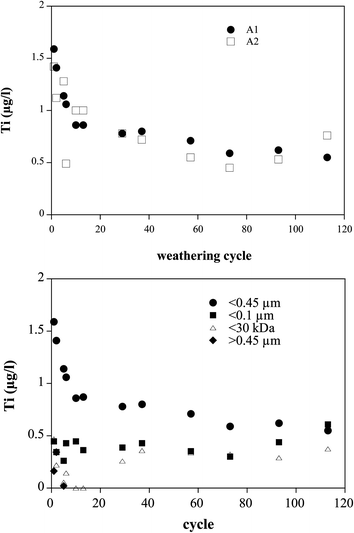 | ||
| Fig. 3 Top: release of TiO2 particles from painted panels A1 (nano-TiO2 and pigment-TiO2) and A2 (pigment-TiO2) in the weathering chamber. Bottom: size fractionation of TiO2 released from paint A1. | ||
The size fractionation of Ti released from A1 is given in Fig. 3b. The fraction below 100 nm was more or less constant throughout the test, the higher initial concentrations were caused by higher amounts of particles between 100 and 450 nm. The dissolved Ti was about equal to the fraction smaller than 100 nm. Digests of the 0.45 μm filters showed that the Ti-content of the particles larger than 450 nm was below 0.2 μg l−1 and therefore constituted not an important Ti-fraction compared to the smaller particles.
Electron microscopic investigations of panels
SEM-EDX investigations were performed for the panels painted with A1 and A2 before and after weathering. A representative image of panel A1 is given in Fig. 4. TiO2 particles were identified by EDX analysis and found to be well spread within the paint matrix. They were embedded and covered with a binder. EDX analysis on several points revealed mainly a Ca signal. TiO2 particles appear in a spherical form that ranged from 90 nm to 200 nm and were strongly agglomerated.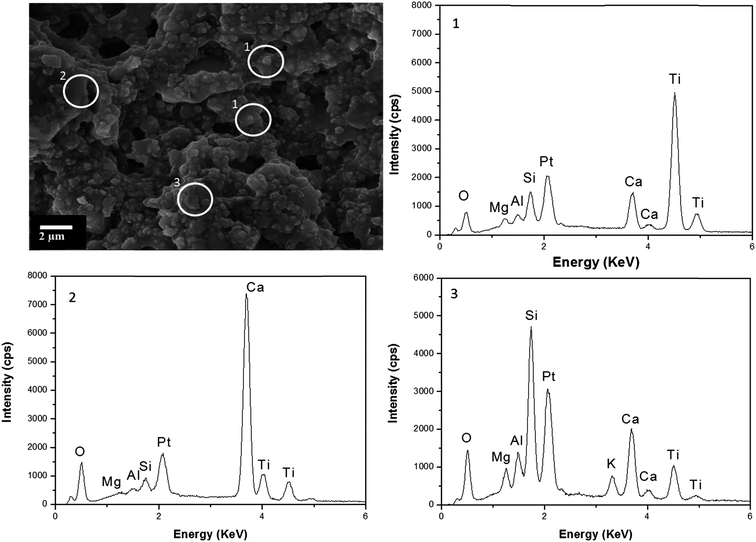 | ||
| Fig. 4 SEM image of panel A1 before weathering. The EDX spectra of selected area are at the right side. | ||
The effect of weathering was investigated by SEM on the same panels after the experiments (Fig. 5). The images show that the polymer layer was partially destroyed, but the TiO2 particles remained attached to the surface.
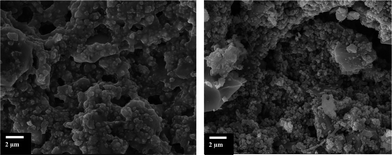 | ||
| Fig. 5 Effects of the weathering experiments on panel A1. On the left side the panels before weathering and on the right side the panels after weathering. The TiO2 particles embedded in the binder before weathering are completely or partially exposed after weathering. | ||
Electron microscopy of leachates
Particles in aliquots from the leachate were deposited on TEM grids and investigated using TEM. Due to the very low Ti-concentration in the leachates (less than 1.5 μg l−1), no Ti-containing particles could be found in any of the investigated leachate samples.Laboratory tests
The influence of various experimental parameters on the release of TiO2 particles from the paints was investigated in a smaller test setup in a batch experiment (Fig. 6). The following parameters were varied: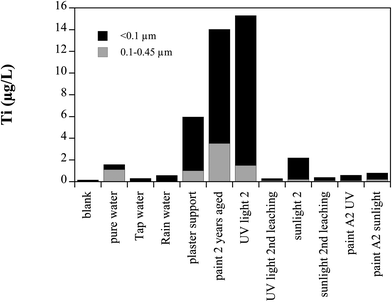 | ||
| Fig. 6 Release of TiO2 particles from small panels (10 × 10 cm) under different conditions. The standard condition is paint A1 applied on fiber cement leached in pure water. Only derivations from this condition are noted in the figure. Shown are the average values of 2 or 3 replicates. | ||
(a) media used (deionized water, tap water and rain water)
(b) support used (fiber cement or plaster)
(c) illumination (UV lamp, sunlight)
(d) the composition of the paint (paint A1, paint A1 stored for 2 years, paint A2)
(e) leaching a second time.
With pure water, tap water and rain water less than 2 μg l−1 Ti was leached from A1 on fiber cement. The same paint on plaster leached 6 μg l−1 into pure water. The painted fiber panel stored for 2 years in the dark before leaching released 14 μg l−1 Ti. Freshly painted samples exposed to UV light for 3 weeks leached 15 μg l−1. Exposed to sunlight for 3 weeks the same paint only leached 2 μg l−1. The paint A2 released less than 1 μg l−1 of Ti. Panels that were leached the first time and then exposed to the same conditions (UV light and sunlight) leached almost no Ti in the second leaching. In all samples the majority of Ti was in the fraction smaller than 100 nm.
Extraction of aged paint
The aged and milled paints of A1 and A2 were extracted in water and the stable fraction in suspension was measured after 24 h of sedimentation (Fig. 7). The amounts of Ti released from A1 are with 80 μg l−1 much higher compared to the nanoparticle-free paint A2 with 6 μg l−1 that is free of ENM. About 60% of Ti in the A1 extract was present in the fraction 100–450 nm, 35% in the particulate fraction smaller than 100 nm. TiO2 in the stable suspension represents about 0.025% of the total TiO2 (pigment and nano-TiO2) in the added paint.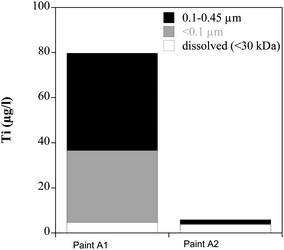 | ||
| Fig. 7 Extraction of Ti from 2 g l−1 of aged and milled paints A1 and A2 with distilled water. | ||
Discussion
In this work two paints of the same composition and the same total TiO2 content were used, but one containing part of TiO2 in the form of nano-TiO2, the other only pigment-TiO2. Both paints released only very low amounts of Ti into the leachate during the climate chamber experiments, which represent an accelerated weathering compared to natural conditions.27 Even the first leachate sample contained only 1.5 μg l−1 Ti released from the paint, a concentration only slightly above the background Ti concentration in the used leaching water. The observed concentrations are many orders of magnitude lower than those Ti-concentrations in a nano-Ag containing paint leachate (up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg l−1 in the first sample)16 and in the run-off from house facades (300–600 μg l−1).14 These two studies exposed the paints to outdoor conditions whereas we have used controlled climate chambers that, however, are representative for natural conditions and correspond to an accelerated weathering. Due to the very low μg l−1 concentrations of particles, none could be observed by TEM and thus the identity of the released Ti remains open. The fractionation clearly showed that particulate Ti prevailed, suggesting that indeed TiO2 was released. In the first 10 weathering cycles the released Ti decreased by about 50% and then stabilized. Such a trend was not observed for the matrix elements of paint A1 but for Ca in paint A2. In the mentioned outdoor study on nano-Ag paint16 the released Ag and Ti decreased exponentially over many months. Compared to these results the decrease observed in our study is almost non-existent, again indicating that Ti was strongly fixed in the paint matrix. A small amount of dissolved Ti (smaller than 30 kDa) was detected. Although the identity of this species is unknown, dissolved Ti was found in other studies to be an important species. In a swimming pool water almost all Ti was present in the dissolved form28 and also in natural water affected by human influence up to 70% of Ti was found in the dissolved form.29
000 μg l−1 in the first sample)16 and in the run-off from house facades (300–600 μg l−1).14 These two studies exposed the paints to outdoor conditions whereas we have used controlled climate chambers that, however, are representative for natural conditions and correspond to an accelerated weathering. Due to the very low μg l−1 concentrations of particles, none could be observed by TEM and thus the identity of the released Ti remains open. The fractionation clearly showed that particulate Ti prevailed, suggesting that indeed TiO2 was released. In the first 10 weathering cycles the released Ti decreased by about 50% and then stabilized. Such a trend was not observed for the matrix elements of paint A1 but for Ca in paint A2. In the mentioned outdoor study on nano-Ag paint16 the released Ag and Ti decreased exponentially over many months. Compared to these results the decrease observed in our study is almost non-existent, again indicating that Ti was strongly fixed in the paint matrix. A small amount of dissolved Ti (smaller than 30 kDa) was detected. Although the identity of this species is unknown, dissolved Ti was found in other studies to be an important species. In a swimming pool water almost all Ti was present in the dissolved form28 and also in natural water affected by human influence up to 70% of Ti was found in the dissolved form.29
The 1.5 μg l−1 in the first leachate in the climate chamber correspond to 0.22 Ti μg g−1 of paint, taking into account the volume of leachate water and the weight of the paint exposed. In the small-scale leaching experiments the released Ti was 0.05–1.3 μg g−1, with pure water and UV-exposed panels as the two extremes. The two types of experiments therefore yielded similar release rates and can be used to decipher some of the factors that control the release. Whereas the type of water did not influence the release, the type of support did. The climate chamber support was fiber cement, having a smooth surface. Applying the paint on plaster resulted in 4-times higher release. These samples had a rough surface and therefore much higher surface area of paint was exposed to the water.
A strong increase in release of Ti was observed when the small panels were exposed to UV-light, indicating that photocatalytic degradation of the paint matrix by the nano-TiO2 is responsible for the release. The small panels were much longer exposed to UV-light than in the climate chamber – 3 weeks compared to 3 hours per cycle – and thus a stronger effect can be expected. The paint with only pigment-TiO2 did not show this increase in release after UV-illumination. Also the aged and milled paint showed a clear difference between the nano-containing and the pigment-only paint, with a release of Ti of 40 μg g−1, about a factor of 40–200 more than from the painted panels. Due to the milling the exposed surface area of this sample was much higher and the difference between the two paints became much more visible.
It is well known that the organic paint matrix can be partially degraded by the photocatalytic activity of TiO2. During this process the photocatalytic activity of the paints towards organic pollutants was increased.30,31 This was explained by the removal of the organic binder from the surface of TiO2, exposing more of the photoactive particles on the surface of the paint. This removal of the organic binder was also visible in the electron microscopic analysis of the paint surface after the climate chamber exposure. A certain increase in release of TiO2 was observed, however, the degradation of the paint was obviously not strong enough to result in more than a few particles to be detached. Even after an UV-illumination that corresponds to about 1 year of outdoor exposure no increase in release of Ti was found, with the small panels a second UV-exposure and leaching released not more particles than leaching without illumination. The process of destruction of the polymer is therefore not a linear and continuous process but seems to stop or pause for at least the time studied in this work. Exposure of CNT-containing polymers to UV-irradiation resulted in degradation of the polymer and formation of a protecting layer of CNTs on the surface, slowing down the further degradation of the polymer.32 It is possible that a similar process is also at work in the paint studied in this work.
For estimating the risks of nano-TiO2 to the environment, it is necessary to consider the whole life-cycle of the product.33 Based on our work we can state that the used paints remained stable for a time representative of one year of outdoor exposure. The results would need to be extrapolated to 20 years, the usual time when an outdoor paint is renewed.34 A simple linear extrapolation of our results to 20 years is not feasible, as continued UV-exposure may further degrade the paint matrix and eventually result in release of TiO2. To study this, controlled long-term outdoor exposure experiments would need to be performed. At the moment we can only state that release of TiO2 from outdoor paints seems to be low and is probably not a major mass flow of nano-TiO2 into the environment.35 There are several design options available for hazard reduction: better fixation of the particles in the composites, including sustained suppression of oxidative damage to the polymer, changes of particle surface, structure or composition, and design changes that result in the release of relatively large particles.36
Acknowledgements
This work was founded by the European Commission within the Seventh Framework Program (FP7; NanoHouse project – Grant Agreement no. 247810). We thank Aron Christofel for help with the experiments.References
- M. R. Wiesner, G. V. Lowry, K. L. Jones, M. F. Hochella, R. T. Di Giulio, E. Casman and E. S. Bernhardt, Environ. Sci. Technol., 2009, 43, 6458–6462 CrossRef CAS.
- M. Farre, J. Sanchis and D. Barcelo, Trends Anal. Chem., 2011, 30, 517–527 CrossRef CAS PubMed.
- J. R. Peralta-Videa, L. J. Zhao, M. L. Lopez-Moreno, G. de la Rosa, J. Hong and J. L. Gardea-Torresdey, J. Hazard. Mater., 2011, 186, 1–15 CrossRef CAS PubMed.
- S. J. Klaine, A. A. Koelmans, N. Horne, S. Carley, R. D. Handy, L. Kapustka, B. Nowack and F. von der Kammer, Environ. Toxicol. Chem., 2012, 31, 3–14 CrossRef CAS PubMed.
- A. Baun, N. B. Hartmann, K. Grieger and K. O. Kusk, Ecotoxicology, 2008, 17, 387–395 CrossRef CAS PubMed.
- T. M. Scown, R. van Aerle and C. R. Tyler, Crit. Rev. Toxicol., 2010, 40, 653–670 CrossRef CAS PubMed.
- F. Gottschalk and B. Nowack, J. Environ. Monit., 2011, 13, 1145–1155 RSC.
- M. Hassellov and R. Kaegi, in Environmental and Human Health Impacts of Nanotechnology, ed. J. R. Lead and E. Smith, Blackwell Publishing Ltd., 2009, ISBN: 978-1-405-17634-7 Search PubMed.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS PubMed.
- B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans and S. J. Klaine, Environ. Toxicol. Chem., 2012, 31, 50–59 CrossRef CAS PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- J.-P. Kaiser, S. Zuin and P. Wick, Sci. Total Environ., 2013, 442, 282–289 CrossRef CAS PubMed.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS PubMed.
- A. Weir, P. Westerhoff, L. Fabricius, K. Hristovski and N. von Götz, Environ. Sci. Technol., 2012, 46, 2242–2250 CrossRef CAS PubMed.
- R. Kaegi, B. Sinnet, S. Zuleeg, H. Hagendorfer, E. Mueller, R. Vonbank, M. Boller and M. Burkhardt, Environ. Pollut., 2010, 158, 2900–2905 CrossRef CAS PubMed.
- B. C. Reinsch, C. Levard, Z. Li, R. Ma, A. Wise, K. B. Gregory, G. E. Brown and G. V. Lowry, Environ. Sci. Technol., 2012, 46, 6992–7000 CrossRef CAS PubMed.
- J. Olabarrieta, S. Zorita, I. Pena, N. Rioja, O. Monzon, P. Benguria and L. Scifo, Appl. Catal., B, 2012, 123–124, 182–192 CrossRef CAS PubMed.
- A. T. Saber, I. K. Koponen, K. A. Jensen, N. R. Jacobsen, L. Mikkelsen, P. Möller, S. Loft, U. Vogel and H. Wallin, Nanotoxicology, 2011, 1–13 Search PubMed.
- I. K. Koponen, K. A. Jensen and T. Schneider, J. Phys.: Conf. Ser., 2009, 151, 012048 CrossRef.
- D. E. Jacobs, R. P. Clickner, J. Y. Zhou, S. M. Viet, D. A. Marker, J. W. Rogers, D. C. Zeldin, P. Broene and W. Friedman, Environ. Health Perspect., 2002, 110, A599–A606 CrossRef CAS.
- H. W. Mielke and P. L. Reagan, Environ. Health Perspect., 1998, 106, 217–229 CAS.
- J. Lee, S. Mahendra and P. J. J. Alvarez, ACS Nano, 2010, 4, 3580–3590 CrossRef CAS PubMed.
- C. Suryanarayana, Prog. Mater. Sci., 2001, 46, 1–184 CrossRef CAS.
- EOTA, ETAG 004 – Guideline for European Technical Approval of External Thermal Insulation Composite Systems with Rendering, Edition March 2000, European Organisation for Technical Approvals, Brüssel, http://www.eota.be/en-GB/content/endorsed-etag-s/9/, 2000 Search PubMed.
- L. Windler, C. Lorenz, N. Von Goetz, H. Hungerbuhler, M. Amberg, M. Heuberger and B. Nowack, Environ. Sci. Technol., 2012, 46, 8181–8188 CrossRef CAS PubMed.
- T. P. Wangler, S. Zuleeg, R. Vonbank, K. Bester, M. Boller, J. Carmeliet and M. Burkhardt, Build. Sci., 2012, 54, 168–173 Search PubMed.
- R. David Holbrook, D. Motabar, O. Quiñones, B. Stanford, B. Vanderford and D. Moss, Environ. Pollut., 2013, 181, 68–74 CrossRef CAS PubMed.
- C. Neal, H. Jarvie, P. Rowland, A. Lawler, D. Sleep and P. Scholefield, Sci. Total Environ., 2011, 409, 1843–1853 CrossRef CAS PubMed.
- T. Marolt, A. S. Skapin, J. Bernard, P. Zivec and M. Gaberscek, Surf. Coat. Technol., 2011, 206, 1355–1361 CrossRef CAS PubMed.
- P. A. Christensen, A. Dilks, T. A. Egerton and J. Temperley, J. Mater. Sci., 1999, 34, 5689–5700 CrossRef CAS.
- T. Nguyen, B. Pellegrin, C. Bernard, X. Gu, J. M. Gorham, P. Stutzman, D. Stanley, A. Shapiro, E. Byrd, R. Hettenhouser and J. Chin, J. Phys.: Conf. Ser., 2011, 304, 012060 CrossRef.
- C. Som, M. Berges, Q. Chaudhry, M. Dusinska, T. F. Fernandes, S. I. Olsen and B. Nowack, Toxicol., 2010, 269, 160–169 CrossRef CAS PubMed.
- HEV, Table of Building Components Service Life by Swiss Home Owners' Federation (HEV), http://www.hev-schweiz.ch, 2009 Search PubMed.
- R. Arvidsson, S. Molander and B. A. Sandén, J. Ind. Ecol., 2012, 16, 343–351 CrossRef CAS.
- L. Reijnders, Polym. Degrad. Stab., 2009, 94, 873–876 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
