 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mercury dynamics in groundwater across three distinct riparian zone types of the US Midwest
Philippe G. Vidon*a, Carl P. J. Mitchellb, Pierre-André Jacinthec, Matthew E. Bakerd, Xiaoqiang Liue and Katelin R. Fishere
aDepartment of Forest and Natural Resources Management, The State University of New York College of Environmental Science and Forestry (SUNY-ESF), 1 Forestry Drive, Syracuse, NY 13210, USA. E-mail: pgvidon@esf.edu; Fax: +1 315 4706535; Tel: +1 315 470 4765
bDepartment of Physical and Environmental Sciences, University of Toronto Scarborough, Toronto, ON, Canada
cDepartment of Earth Sciences, Indiana University-Purdue University Indianapolis (IUPUI), Indianapolis, IN, USA
dDepartment of Geography and Environmental Systems, University of Maryland Baltimore County (UMBC), Baltimore, MD, USA
eDepartment of Earth Sciences, Indiana University-Purdue University Indianapolis (IUPUI), Indianapolis, IN, USA
First published on 4th October 2013
Abstract
Although the intense biogeochemical gradients present in riparian zones have the potential to affect mercury (Hg) cycling, Hg dynamics in riparian zones has received relatively little attention in the literature. Our study investigated groundwater filtered total mercury (THg) and methylmercury (MeHg) dynamics in three riparian zones with contrasting hydrogeomorphic (HGM) characteristics (till, alluvium, outwash) in the US Midwest. Despite high Hg deposition rates (>16 μg m−2) in the region, median THg (<1.05 ng L−1) and MeHg (<0.05 ng L−1) concentrations were low at the study sites. Methylmercury concentrations were significantly (p < 0.05) correlated to THg (R = 0.82), temperature (R = 0.55), and dissolved organic carbon (DOC) (R = 0.62). THg also correlated with groundwater DOC (R = 0.59). The proportion of MeHg in THg (%MeHg) was significantly correlated to temperature (R = 0.58) and MeHg (R = 0.50). Results suggest that HGM characteristics, the presence of tile drains, and the propensity for overbank flooding at a riparian site determined the extent to which stream water Hg concentrations influenced riparian groundwater Hg levels or vice versa. Differences in hydrogeomorphic characteristics between sites did not translate however in significant differences in groundwater MeHg or %MeHg. Overall, widespread Hg contamination in the most common riparian hydrogeomorphic types of the US Midwest is unlikely to be a major concern. However, for frequently flooded riparian zones located downstream from a potentially large source of Hg (e.g., concentrated urban development), Hg concentrations are likely to be higher than at other sites.
Environmental impactAlthough mercury (Hg) is a major environmental contaminant, Hg dynamics in riparian zones has received little attention. Our study revealed that in the US Midwest, filtered total mercury (THg) and methylmercury (MeHg) concentrations in riparian groundwater were low in spite of high Hg deposition rates. Landscape hydrogeomorphic characteristics, the presence of tile drains, and the propensity for overbank flooding at a riparian site determined the extent to which stream water Hg concentrations influenced riparian groundwater Hg or vice versa. Differences in hydrogeomorphic characteristics between sites did not translate however in significant differences in groundwater MeHg or %MeHg in THg. However, at frequently flooded sites downstream from potentially large Hg sources, Hg concentrations are likely to be higher than at other sites. |
Introduction
Mercury (Hg) is a potent neurotoxin to wildlife and humans, and Hg contamination of freshwater resources is a major concern nationwide.1,2 In 2009, 43% of US lakes were under Hg fish consumption advisory, while in 2006, 80% of all state fish consumption advisories were related to excessive Hg concentration in fish.3,4 Many studies have therefore investigated the distribution of Hg in streams,5 whole catchments,6 wetlands,7,8 and lakes.9,10 These studies have documented a strong association between Hg and organic matter in surface waters, leading to the general assumption that ecosystems with highly organic soils (e.g., wetlands) are areas of preferential mercury accumulation.11,12 In addition, because of the high toxicity and bioaccumulation potential of methylmercury (MeHg), processes driving MeHg production and distribution in the environment are of outmost concern.10,13 Studies have shown that hot spots of MeHg production often involve water-saturated areas characterized by significant amounts of sulfate reduction.7 Generally, Hg methylation is associated with the presence of organic matter, anoxic conditions, and an adequate supply of sulfate to sustain the activity of sulfate-reducing bacteria.11,14Although a few studies have investigated Hg transport in riparian zones, relatively little information is available in the literature about the distribution of Hg and/or MeHg in riparian zones.15,16 In regions of significant atmospheric deposition, or at locations susceptible to Hg contamination from upstream urban sources, riparian zones have nevertheless the potential to play an important role in regulating the fate and transport of both Hg and MeHg in the environment.3,17 Indeed, riparian zones act as both a buffer and a preferential conduit for solutes from the upland environment to the stream,18 and often include soils that are wetter and have higher organic matter content than their upland counterparts.19 Strong biogeochemical gradients have also been reported in riparian zones20–23 and in particular, significant sulfate reduction where persistent anoxic conditions exist.19,24 In riparian zones receiving significant amount of Hg, either via atmospheric deposition or riverine processes, the co-occurrence of Hg delivery, organic matter accumulation, and anoxia could potentially lead to significant amounts of Hg accumulation in association with organic matter, and potentially high Hg methylation rates.
In many studies, researchers have demonstrated that riparian water table dynamics, stream–riparian interactions, and soil organic carbon content can vary drastically depending on soil texture, depth to a confining layer (e.g. glacial till, bedrock, clay), and topography.25–28 This implies that Hg accumulation and MeHg production in subsurface waters may also vary significantly with riparian hydrogeomorphology. However, no study has systematically assessed the importance of hydrogeomorphologic variation on Hg and MeHg dynamics in riparian groundwater. This information gap strongly hinders our ability to generalize riparian zone functioning with respect to Hg, and ultimately our ability to better constrain sinks and sources of Hg and MeHg in the landscape for management purposes.
In this study, we investigated filtered total mercury (THg) and MeHg concentrations in the groundwater of three riparian zones with contrasting hydrogeomorphic characteristics in Indiana, USA, where there exists relatively little information on riverine or riparian mercury cycling despite relatively high Hg deposition rates (>16 μg m−2).29 Subsurface biogeochemical conditions at each site (temperature, oxidation–reduction potential, sulfate and dissolved organic carbon concentrations) were measured along with the collection of subsurface water samples for Hg analysis to better understand factors regulating Hg levels and whether these riparian areas have substantial capacity to methylate Hg. The primary objectives of the study were (1) to determine the primary drivers of THg and MeHg concentrations in groundwater across sites, (2) to determine the primary variables regulating Hg methylation at each site, and (3) to assess the extent to which hydrogeomorphic characteristics can be used to generalize riparian functioning with respect to THg and MeHg in the landscape.
Materials and methods
Site description
Three riparian zones with contrasting hydrogeomorphic characteristics were selected near Indianapolis, IN (Fig. 1). Climate in Indianapolis is classified as temperate continental and humid. Annual precipitation is 104 cm per year with approximately 6.9 cm falling as snow between November and March.30 During the duration of the study, precipitation amounts were 6% wetter than the 30 year normal.31 The mean annual temperature is 11.4 °C with a mean January temperature of −3.1 °C and a mean July temperature of 24.1 °C. The growing season, which is defined as the last and first freeze date over a 30 year period, extends from April 10 until October 28.30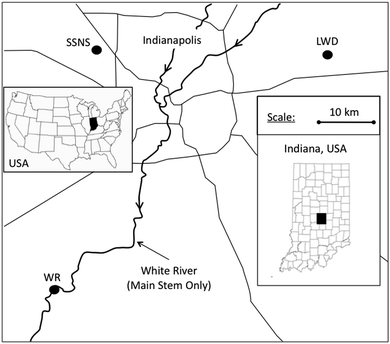 | ||
| Fig. 1 Location of the Leary Weber Ditch site (LWD), Scott Starling Nature Sanctuary site (SSNS), and White River site (WR) in Indiana, USA. | ||
The three riparian zones studied were selected to represent the three dominant riparian hydrogeomorphic types commonly found in glaciated landscapes of the upper Midwest. The Leary Weber Ditch riparian area (LWD) is located approximately 30 km east of Indianapolis. This site represents the narrow riparian zones (20–30 m wide) that are prevalent along tile-drained corn (Zea mays) and soybean (Glycine max) fields in till plains of the US Midwest. Soils at the LWD site are poorly-drained and belong to the Crosby–Brookston association. In the riparian zone, silty clay loam soil can be found in the upper 1.2 m to 1.5 m of the soil profile. Subsurface drains (a.k.a. tile drains) are located approximately 1.3 m below the ground surface in this grass dominated riparian zone (Fig. 2). The stream at this site is deeply incised (2.5 m deep) and has periodically been dredged to prevent crop fields from flooding. Further details about this site are available in Vidon and Cuadra (2010).32
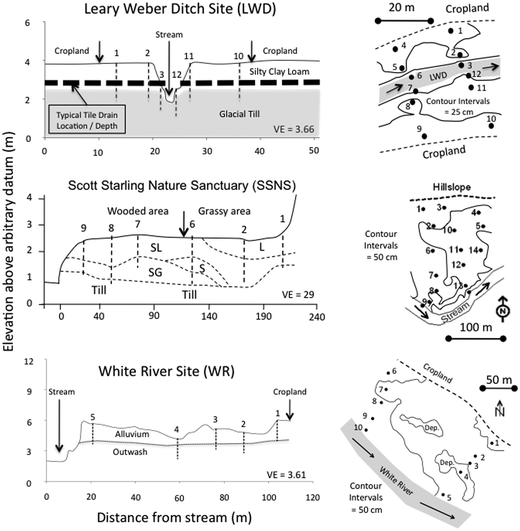 | ||
| Fig. 2 Cross-sectional view of the Leary Weber Ditch (LWD), Scott Starling Nature Sanctuary (SSNS), and White River (WR) sites indicating soil texture or sediment type, and piezometer and well locations (left panel). Planar view of the LWD, SSNS, and WR sites indicating the location of well and piezometer nests (number 1 to 14) at each site, as well as general site topography (right panel). (Legend: L = loam; SL = sandy loam; S = sand; SG = sandy gravel; VE = vertical exaggeration.) | ||
The Scott Starling Nature Sanctuary riparian zone (SSNS) is located approximately 10 km west of Indianapolis and corresponds to riparian zones found at the outlet of second to fourth order streams in deep (15–20 m) and wide river valleys (100–200 m) incised into the till plain.33 Thin outwash deposits and shallow layers of alluvium (1–2 m) are often found in these riparian zones, where land use in the adjacent upland is generally dominated by managed forest and/or low density residential housing development. At SSNS, soils developed in a thin layer of alluvium (approx. 2 m deep) overlying a confining layer of glacial till that restricts subsurface flow to the first 2 m of the soil profile. Dominantly loamy soil can be found near the hillslope where grassy vegetation dominates, whereas sandy loam and gravelly sandy loam soils can be found closer to the stream in the wooded section of the riparian zone (Fig. 2). Historically, this site was artificially drained and used for agriculture until 1990. Subsurface tile drains were removed in 1999–2004 and wetland hydrology restored near the hillslope where grassy vegetation has since re-established.33,34
The third site is the White River riparian zone (WR), located approximately 50 km south of Indianapolis along the banks of the White River. This site corresponds to large riparian zones (100–200 m wide) located along 4th order streams and larger channels where outwash and/or alluvium deposits have accumulated since the last glacial maximum. Soils at the WR site are well drained, and consist of approximately 2 m of silt loam soil (alluvium) overlying a gravel layer and outwash deposits at greater depth (Fig. 2). This wooded riparian zone (approx. 100 m wide) is often flooded in response to changes in water levels in the adjacent river. Land-use in the upland is agricultural with a corn–soybean rotation.
Methods
Three transects of wells and piezometers were installed at the SSNS site, and two transects were installed at the WR and LWD sites. Each transect is composed of 5 to 6 nests of piezometers and wells (Fig. 2). Each nest is composed of one PVC well (5 cm diameter, PVC, screened on entire depth, 130 cm to 200 cm deep depending on site), and piezometers (1.74 cm diameter, PVC, 20 cm slotted end) installed every 50 cm from the ground surface down to the confining layer at the LWD and SSNS site, and down to a depth of 2 m at the WR site. A nylon mesh sleeve was placed over screened intervals to minimize obstruction of wells and piezometers by fine grain particles. To prevent contamination from surface runoff, holes were filled with sand along the screened intervals and a bentonite clay seal was applied near ground level for wells, or above the screened area for piezometers.35,36 Riparian soil texture was first determined by hand texturing analysis during well and piezometer installation, and later by dry sieving in the laboratory to separate the sand fraction from the clay/silt fraction. Topography was determined at each site using a total station (TC 605L Surveying TPS – Leica Geosystems).Between October 2009 and August 2011, water levels were continuously recorded using a level logger (Solinst, Inc.) in well 1 at LWD, in well 11 at SSNS, and in well 4 at WR (Fig. 2). Over this period, water levels in all piezometers and wells were also recorded manually approximately once a month. All water levels are reported in centimeters below the mean ground surface elevation (cm BMGSE). Water samples for mercury analysis (see sampling methodology below) were collected in all wells at each site in April, July, August, and December 2010, and in February 2011. At the time of groundwater sampling, temperature and oxidation–reduction potential (ORP) in wells were measured in the field using a YSI-600 XLM multi-parameter probe. Water samples in both wells and piezometers were also collected for sulfate (SO42−) and dissolved organic carbon (DOC) concentration analyses in April, July, August, and December 2010, and in February 2011. After collection, all water samples for SO42− and DOC were kept in a cooler at 4 °C until return to the laboratory, where they were immediately filtered using 0.7 μm GF/F filters (Whatman Inc.). Sulfate was analyzed using a photometric analyzer (Aquakem 20, EST Analytical, Fairfield, OH) by EPA standard colorimetric method 375.4.37 Filtered water samples were analyzed for DOC using a Vario Cube TOC analyzer (Elementar Inc., NJ) equipped with a non-dispersible infrared (NDIR) detector. Inorganic C was removed through internal sample acidification (0.1 M H3PO4) and sparging with hydrocarbon-free air. For both sulfate and DOC analyses, triplicate analyses were run on 10% of the samples, and check-standards analyzed every 10 samples. Samples with concentrations below the detection limit were assigned a value equal to one-half the detection limit.
Water samples for Hg analysis were collected using ultra-clean methods (EPA Method 1669). Briefly, using a peristaltic pump and Teflon tubing, well water was pushed through an acid-washed, in-line Teflon filter holder packed with an ashed glass fiber filter (0.7 μm pore size), directly into clean PETG sample bottles. A “clean hands” operator only handled an inner plastic zipper bag and the sample bottle itself while a “dirty hands” operator operated the pump and handled the outer plastic zipper bag in which sample bottles were kept. Samples were kept on ice in the field and then acidified to 0.5% by volume with concentrated, trace-metal grade hydrochloric acid (HCl) in the laboratory within 12 hours of collection. Samples were then stored in the dark at 4 °C until analysis.
Samples for THg concentration were analyzed by cold vapor atomic fluorescence spectroscopy on a Tekran 2600 system following oxidation of samples overnight by the addition of 0.5% by volume of bromine monochloride (BrCl) the day before analysis (EPA method 1631). In this method, all Hg species in water samples are reduced to Hg0 by mixing the sample with SnCl2, and Hg0 is trapped sequentially on dual gold traps, and thermally desorbed into the fluorescence spectrometer. Samples for MeHg concentration were analyzed by isotope dilution-gas chromatography-inductively couple plasma mass spectrometry (ID-GC-ICPMS) according to the methods of Hintelmann and Evans (1997).38 Briefly, water samples were distilled with a known spike quantity of enriched Me199Hg in each sample.39 Distillates were buffered and then ethylated with sodium tetraethylborate in bubblers. Volatile Hg species, including the ethylated MeHg, were trapped onto Tenax-filled glass traps. The Tenax traps were thermally desorbed on a stream of argon, Hg species were separated by gas chromatography, and Hg isotopes were detected by ICPMS. 202Hg was used to calculate ambient MeHg concentrations in relation to the Me199Hg spike. For control of analytical quality, duplicates and matrix spikes were included approximately every 10–15 samples for THg analysis, and duplicates were included approximately every 10–15 samples for MeHg analysis. Mean (±standard deviation) relative standard deviations (RSD) of duplicates were 2.5 ± 2.4% (n = 15) for THg and 6.8 ± 5.7% (n = 9) for MeHg. Matrix spike recoveries were 100 ± 4% (n = 11) for THg. The 199Hg/202Hg ratio RSD of standards used for analyzing the precision of the isotope dilution MeHg analysis was 1.4%.
All statistical analyses were conducted using SAS 9.2. Concentration values were tested for normality using the Shapiro–Wilks W test. Hg data generally were not normally distributed. Log transformation was successful in normalizing THg and %MeHg data, but not MeHg concentrations. Differences among sites for THg and %MeHg were examined by analysis of variance (ANOVA) on log-transformed data, with Tukey post hoc testing where ANOVA tests were significant. The non-parametric Wilcoxon test was used with MeHg concentration data. Pearson product–moment correlation coefficients were used to identify correlation between groups of variables.
Results
Site hydrology
Continuous water level measurements at the LWD, SSNS, and WR sites showed two contrasting time periods during the year, with relatively low water table conditions in late summer and fall, and higher water table conditions during winter, spring, and early summer (Fig. 3). Although all three sites exhibited this seasonal high and low water table pattern, the three study sites exhibited contrasting water table dynamics over the study period (Fig. 3). At the LWD site, water table levels fluctuated between 190 cm BMGSE in December 2010, and 20 cm BMGSE in July 2010 in the riparian zone (excluding wells 3, 6, 7, and 12 located near the bottom of the ditch; Fig. 2). Over the study period, the mean water table depth at the LWD site was 127 cm BMGSE in the riparian area. At the SSNS site, water table fluctuations were less flashy and of a lesser magnitude (range = 117 cm) than at LWD, and the average water table depth over the entire study period (69 cm BMGSE) was higher than at LWD. On the other hand, water table fluctuations at the WR site were more pronounced compared to the other sites, with a water table as low as 240 cm BMGSE on October 2010, and water levels as high as 215 cm above the mean ground elevation in April 2011. Between October 2009 and August 2011, the WR site was flooded by high stream water levels on 5 occasions for at least 24 hours (Fig. 3). Over the entire study period, the mean water table depth at the site (158 cm BMGSE) was lower than at either LWD or SSNS.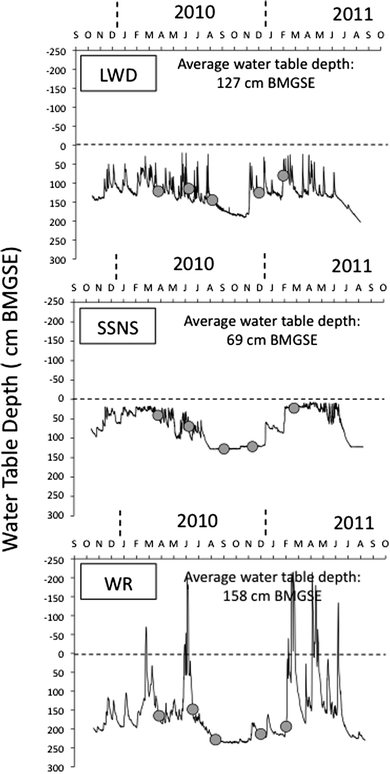 | ||
| Fig. 3 Water table depth in centimeters below the mean ground surface elevation (cm BMGSE) at the Leary Weber Ditch (LWD), Scott Starling Nature Sanctuary (SSNS), and White River (WR) sites between October 2009 and August 2011. Solid black dots indicate the dates when water samples were collected for mercury analysis. The horizontal dotted line indicates the mean ground elevation at each site. | ||
Groundwater biogeochemistry
Mean (±standard deviation) groundwater dissolved organic carbon (DOC) concentrations at the LWD, SSNS, and WR were 8.1 ± 2.26 mg L−1, 5.3 ± 2.24 mg L−1, and 8.3 ± 4.28 mg L−1, respectively (Table 1). Mean groundwater temperature (range of means = 11.5–12.6 °C), ORP (range of means = +59 to +100 mV), SO42− concentrations (range of means = 29.6–31.3 mg L−1) were not significantly different among the study sites (p > 0.05; Table 1). Nonetheless, seasonal variations in these parameters were observed. Groundwater temperature was lowest in February (5.7–7.0 °C) and highest in August (17.4–17.8 °C). Groundwater ORP was generally lower during low water table periods (summer and fall) than during higher water table periods (e.g. spring; Table 1). This trend was particularly visible at LWD and SSNS where ORP was negatively significantly correlated (p < 0.05) with water table depth (R = −0.78 at LWD, R = −0.77 at SSNS). The correlation between ORP and water table depth at the WR site (R = −0.40) was not significant (p > 0.05). SO42− concentrations were not significantly correlated to either temperature or water table level at any of the sites (p > 0.05). However, at all sites, the highest SO42− concentrations were observed during the December sampling date (Table 1). The lowest SO42− values were observed in August at the WR site, and in July at the SSNS and LWD sites (Table 1). Over the study period, groundwater DOC concentrations varied from less than 4 mg L−1 in April 2010 at the SSNS and WR sites, to 7.2 mg L−1 in July at SSNS and 12.3 mg L−1 at WR in August. At the LWD site, DOC concentrations were also higher in July and August (DOC > 9 mg L−1) than in February (DOC = 6.1 mg L−1) or April (DOC = 6.7 mg L−1). When all sampling dates and all sites were considered together, DOC concentration was significantly correlated (p < 0.05) to water table depth (R = 0.59), temperature (R = 0.63), and ORP (R = −0.79).| WT (cm BMGSE) | T (°C) | ORP (mV) | SO42− (mg L−1) | DOC (mg L−1) | ||
|---|---|---|---|---|---|---|
| April | LWD | 122 | 8.1 | 53 (69) | 30.7 (32.4) | 6.7 (1.6) |
| SSNS | 40 | 9.6 | 303 (109) | 18.2 (6.4) | 3.7 (1.2) | |
| WR | 165 | 10.9 | 166 (51) | 25.0 (7.0) | 3.5 (1.4) | |
| July | LWD | 115 | 14.6 | −32 (93) | 26.1 (26.1) | 9.9 (2.1) |
| SSNS | 70 | 18.0 | 75 (156) | 14.6 (10.9) | 7.2 (2.5) | |
| WR | 148 | 16.6 | 17 (124) | 28.9 (15.0) | 9.0 (3.7) | |
| August | LWD | 145 | 17.4 | −67 (70) | 32.5 (41.2) | 9.6 (0.8) |
| SSNS | 127 | 17.8 | −47 (52) | 19.0 (9.7) | — | |
| WR | 228 | 17.7 | −27 (69) | 24.1 (21.5) | 12.3 (3.3) | |
| December | LWD | 126 | 10.3 | 15 (37) | 45.0 (47.1) | — |
| SSNS | 121 | 9.5 | 41 (22) | 66.8 (38.2) | 4.7 (0.9) | |
| WR | 213 | 10.6 | 74 (117) | 36.6 (1.1) | 9.5 (5.9) | |
| February | LWD | 81 | 6.9 | 84 (41) | 29.1 (30.7) | 6.1 (1.2) |
| SSNS | 22 | 5.7 | 130 (16) | 37.9 (15.0) | 5.5 (2.0) | |
| WR | 193 | 7.0 | 67 (8.8) | 34.6 (4.4) | 7.4 (3.0) | |
| All 5 dates | LWD | 118 | 11.5 | 85 (84.6) | 29.6 (32.4) | 8.1 (2.26) |
| SSNS | 76 | 12.1 | 100 (146) | 31.3 (24.5) | 5.3 (2.24) | |
| WR | 190 | 12.6 | 59 (104) | 29.8 (12.3) | 8.3 (4.28) |
Groundwater mercury concentrations
Over the study period, THg concentrations at the WR site (median = 1.05 ng L−1) were significantly higher (p < 0.0001) than at the SSNS site (median = 0.33 ng L−1) and the LWD site (median = 0.28 ng L−1; Fig. 4). The difference in THg concentration between LWD and SSNS was not statistically significant (Fig. 4). Methylmercury concentrations were relatively low and not significantly different (p > 0.05) among the study sites (median concentration of 0.01, 0.03 and 0.05 ng L−1 at LWD, SSNS and WR, respectively). Although differences were not always statistically significant (see above), median THg and MeHg concentrations were typically highest at the WR site, followed by SSNS, and then LWD. With MeHg concentrations expressed as a proportion of the THg concentration (%MeHg), the highest %MeHg was consistently observed at the SSNS site (median = 17% MeHg). Lower %MeHg values were observed at the LWD (median = 9% MeHg) and WR (median = 5% MeHg) sites. The only significant difference in %MeHg among sites was between SSNS and WR (p = 0.029).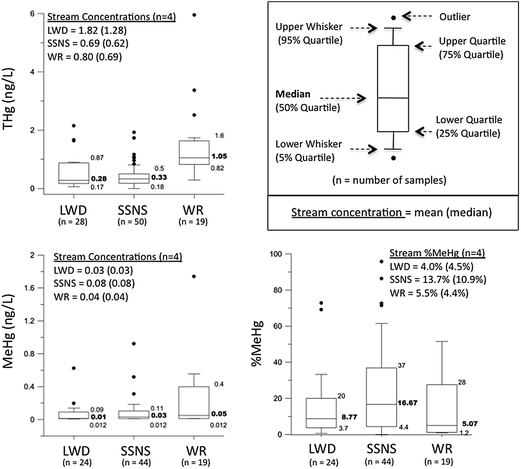 | ||
| Fig. 4 Box plots showing filtered total mercury (THg), methylmercury (MeHg), and the fraction of MeHg in THg (%MeHg) in groundwater at the Leary Weber Ditch (LWD), Scott Starling Nature Sanctuary (SSNS), and White River (WR) sites over the study period. Mean (median) THg, MeHg and %MeHg values in stream water for each site over the study period are also indicated. The number of samples (n value) differs between THg and MeHg because when not enough sample volume was available for both THG and MeHg analyses (e.g. low water table), only THg was analyzed. | ||
Seasonal patterns in THg concentration were not consistent among the study sites (Fig. 5). For instance, at the WR site, a clear increase in groundwater THg concentrations was observed over the course of the growing season (April 10–October 28) with highest THg concentrations observed in August, and lowest concentrations observed in late winter (February). An almost opposite pattern was observed at the SSNS site where the lowest groundwater THg concentrations were observed in August, and highest values in April and July 2010. No clear seasonal pattern in THg was observed at the LWD site. We did however observe clear seasonal MeHg concentration patterns across all sites, with higher MeHg concentrations in July and August, and lower MeHg concentrations at the end of winter (February 2011; Fig. 5). The median %MeHg was highest in July for LWD and SSNS (23% at LWD, 52% at SSNS) and August for WR (25% at WR). %MeHg was lowest toward the end of the winter (February) at SSNS (5%), LWD (2%), and WR (2%), and/or at the beginning of the growing season (April) at the WR site (1%; Fig. 5). When all Hg sampling dates and all sites were considered together, median THg concentration was significantly correlated (p < 0.05) to water table depth (R = 0.60), and groundwater DOC concentration (R = 0.59). Median MeHg concentration was significantly correlated (p < 0.05) to THg (R = 0.82), temperature (R = 0.55), and groundwater DOC concentration (R = 0.62). Finally, %MeHg was significantly correlated (p < 0.05) to groundwater temperature only (R = 0.58).
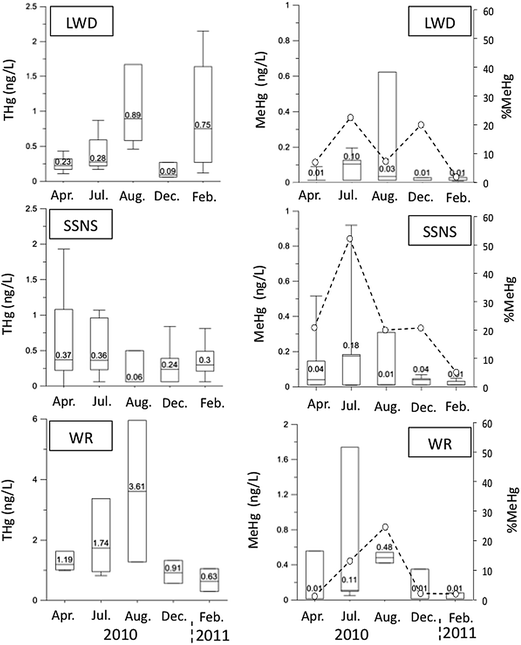 | ||
| Fig. 5 Box plots (5th, 25th, median, 75th, 95th percentiles) showing filtered total mercury (THg) (white bars, left panel), methylmercury (MeHg) (white bars, right panel), and fraction of MeHg in THg (%MeHg) (empty circles and dash lines, right panel) in groundwater at the Leary Weber Ditch (LWD), Scott Starling Nature Sanctuary (SSNS), and White River (WR) sites in April, July, August, and December 2010, and February 2011. Numbers indicate median concentrations of THg and MeHg. | ||
When median THg concentration in stream water and groundwater (Fig. 4) were compared, positive correlations were found between these variables (R = 0.79 at WR, R = 0.58 at LWD, R = 0.39 at SSNS) (Fig. 4). Because of the limited number of stream samples collected (n = 4, no stream Hg samples were collected in April 2010), these correlations were not statistically significant (p > 0.05). With regard to MeHg, however, concentrations in stream water and groundwater were significantly correlated at the WR site (R = 0.85). Correlations between stream and groundwater MeHg were not significant at the LWD site (R = 0.59, p > 0.05), and significantly negative at the SSNS site (R = −0.83, p < 0.05).
Discussion
Landscape controls on Hg concentration in groundwater
Median groundwater THg concentrations (0.28–1.05 ng L−1) across our study sites were lower than has been reported from other studies in the US Midwest region despite relatively high wet atmospheric Hg deposition rates in central Indiana (>16 μg m−2).29 For instance, Stoor et al. (2006) reported THg concentrations between 2 ng L−1 and 14 ng L−1 in groundwater of a forested catchment with sandy soil in upper Michigan.40 In Minnesota and Ontario where wet atmospheric Hg deposition rates (2–4 μg m−2) are considerably lower than in Indiana, Mitchell et al. (2008a) reported median THg concentrations in a series of peatlands between 1.6 and 4.4 ng L−1.7Considering the known affinity of THg for DOC,9 lower THg concentrations at our sites than in other wetland and groundwater environments is likely due to the relatively low DOC concentrations at our sites. Indeed, our sites present relatively low groundwater DOC concentrations (5 mg L−1 < DOC < 9 mg L−1) relative to riparian zones with large organic deposits (e.g. peat, muck) where DOC > 10 mg L−1 are common,23 or many wetland environments (DOC > 15 mg L−1) in Ontario and Minnesota where higher Hg concentrations in pore water have been observed.7 The DOC concentrations at our sites are nevertheless consistent with DOC concentrations (3–5 mg L−1) reported in a wide variety of riparian zones in glaciated settings in Ontario where no large organic deposits (e.g. peat, muck, buried channels) were present,23 with DOC concentrations reported in riparian zones in glacial till valleys of Central New York,41 or with DOC concentrations measured in groundwater under agricultural areas in the Midwest.42,43 Collectively, research suggests that DOC concentrations below 10 mg L−1 in many riparian zones in glaciated settings of the US Midwest, Ontario, or the US Northeast are likely more the norm than the exception. From a management standpoint, unless substantial organic soil deposits are present (e.g., peat, muck, buried channels), this suggests that Hg accumulation and mobility in groundwater may not be a significant environmental issue in many riparian zones located in glaciated settings (as long as point sources of Hg are not present). The significant positive correlation between THg and DOC in groundwater (R = 0.59, p < 0.05) at our sites does suggest that DOC nevertheless plays a critical role in regulating the presence of Hg in solution and, therefore, its mobility. As soil organic carbon is mineralized and DOC concentration increases in groundwater, more THg is released into solution. However, in those glaciated landscapes where soil organic carbon mineralization is low within riparian zones, THg concentrations are similarly low.
When median stream THg or MeHg data (Fig. 4) were compared to median THg or MeHg concentrations in riparian groundwater, stream THg and MeHg concentrations were positively correlated with groundwater THg and MeHg concentrations at all sites (except SSNS for MeHg). Although not all correlations were statistically significant, the results are consistent with our understanding of the hydrologic functioning of the study sites showing a good stream-to-riparian zone connectivity at WR, a good riparian-to-stream connectivity at LWD, and a poor stream–riparian zone connectivity at SSNS. Indeed, previous hydrological studies at SSNS did not show a good connectivity between the stream and riparian zone water at that site.33,44 For example, although groundwater flow direction varies at SSNS as a function of seasons and in response to precipitation events, overbank flooding was never observed at this site over a two-year period.33,44 When flow reversals occur (i.e., stream to riparian zone hydraulic gradient), they do not last long enough to generate any significant input of stream water into the riparian zone.44 This lack of hydrological connectivity is consistent with the weak or negative correlations observed between stream THg and/or MeHg and median groundwater THg and/or MeHg during the study at SSNS.
Conversely, well drained sandy soils, no clear lateral groundwater input at the site, and frequent flooding events lasting more than 24 hours suggest greater connectivity between stream water and riparian groundwater at the WR site where most riparian groundwater directly comes from the stream itself. The relatively high correlation coefficients between stream THg and riparian groundwater THg (R = 0.79, p > 0.05) as well as between stream MeHg and riparian groundwater MeHg (R = 0.85, p < 0.05) are consistent with the stream having a strong influence on Hg distribution in groundwater at that site. This relationship is also consistent with field observations of Hatcher and Filippelli (2011) showing elevated Hg concentration (i.e. >50 ppb) in sediments collected from frequently flooded banks of the White River downstream from Indianapolis, whereas mean sediment Hg concentrations upstream of the city were only 6 ppb.17 At the LWD site, most of the water reaching the stream comes from subsurface drains and strongly resembles groundwater.45–47 The relatively high correlation coefficients between stream and riparian THg (R = 0.58, p > 0.05) and MeHg (R = 0.59, p > 0.05) in the LWD case are consistent with stream water being strongly influenced by groundwater.
Overall, the data suggest that the hydrogeomorphic setting of the riparian zone (i.e. topography, soil type, confining layer depth, subsurface drains, susceptibility to flooding), by regulating stream–riparian connectivity, determines the extent to which stream water Hg concentrations will influence riparian groundwater Hg (e.g. WR) or vice versa (e.g. LWD). For riparian zones located downstream from a potential large source of Hg to stream water (e.g. large urban development) in outwash riparian valleys often subject to flooding (e.g. WR), Hg concentrations are likely to be higher than in other riparian hydrogeomorphic types.
Biogeochemical controls on MeHg concentration in groundwater
Consistent with the relatively low THg concentrations at our sites, MeHg concentrations (median = 0.01–0.05 ng L−1) were also low compared to other studies. MeHg concentrations in groundwater in a coniferous catchment with highly conductive sandy surficial deposits in Michigan varied, for the most part, between 0.05 mg L−1 and 0.6 mg L−1.40 Median %MeHg at our sites (5–17%) were also on the low end of those reported by Selvendiran et al. (2008) in riparian water in a forested watershed in central New York (overall mean = 29%), or of those reported by Stoor et al. (2006) in Michigan (most values were 5–25%MeHg).16,40At our sites where consistently high SO42− (29.6–31.3 mg L−1) and relatively low DOC (5.3–8.3 mg L−1) were observed, MeHg concentration was most significantly correlated (p < 0.05) to THg (R = 0.82), temperature (R = 0.55), and groundwater DOC (R = 0.62). This suggests that for sites within the range of hydrogeomorphic characteristics studied here, MeHg concentration in groundwater is primarily regulated by Hg abundance, and temperature as it influences microbial activity, soil organic carbon mineralization and the release of DOC in groundwater. On the other hand, %MeHg was only significantly correlated (p < 0.05) to temperature (R = 0.58) and MeHg concentration (R = 0.50), and not significantly correlated (p > 0.05) to groundwater DOC concentration (R = 0.15), SO42− concentration (R = −0.27), THg concentration (R = −0.02), or ORP (R = −0.11). The lack of variability in SO42− concentrations at our sites (29.6–31.3 mg L−1) and sub-optimal reducing conditions (ORP > −100 mV) likely explain the lack of correlation between %MeHg, SO42− and ORP. The positive correlation between %MeHg and temperature nevertheless indicates that Hg methylation at the sites is more likely to occur in summer when temperatures are warm, and groundwater ORP conditions tend to be lower (Table 1). Our findings are consistent with other studies reporting higher Hg methylation rates in summer than in cold winter months, and indicative of a strong microbial control on Hg methylation.48
When temperature, ORP, SO42−, and DOC were compared among LWD, SSNS, and WR, no significant differences (p > 0.05) were observed (Table 1). This suggests that differences in site biogeochemistry are minimal, despite clear differences in hydrogeomorphic characteristics and site hydrology (Fig. 2 and 3). A lack of variability in groundwater biogeochemistry among sites is consistent with the statistical similarity of MeHg concentrations (p > 0.05) among sites. Thus, although hydrogeomorphic approaches have proven extremely useful in explaining among-site variation in nitrate (NO3−) removal in glaciated settings, and have been used to generalize the hydrological functioning of riparian zones with respect to nitrogen at the landscape scale,25–28 differences in ORP, SO42− and DOC at our sites were not strong enough to generate clear differences in MeHg production across riparian hydrogeomorphic types.
The similarity of groundwater ORP between SSNS and the other two sites despite consistent differences in water table levels (Fig. 3) is most likely related to the low DOC concentrations (5.3 mg L−1). Oxidation–reduction processes are tied to the availability of both electron donors and acceptors.49 Because decomposition of organic matter in saturated soils or groundwater quickly consumes limited supplies of oxygen, microbes are forced to switch to alternative electron acceptors.20,26 In terrestrial environments where organic matter is the main electron donor, the redox potential drops as organic matter is oxidized and as oxygen, NO3−, and other electron acceptors are reduced in sequence.50 In the absence of large amounts of organic matter, as is the case at SSNS, organic matter decomposition under anoxic conditions may be limited, which ultimately limits the development of strongly anoxic conditions. From a management standpoint, even in locations with significant wet atmospheric Hg deposition rates, landscapes formerly used for agriculture (e.g., SSNS) may have low soil organic carbon (2.1%),51 and therefore low groundwater DOC (e.g. 5.3 mg L−1), and groundwater Hg, MeHg, and %MeHg despite presenting hydrological conditions similar to many wetland environments, which are normally conducive to high MeHg production and accumulation.7,8,13
Conclusions
This study investigated the distribution of THg, MeHg, and %MeHg in the groundwater of three Midwestern riparian zones with contrasting hydrogeomorphic characteristics. In spite of relatively high wet atmospheric Hg deposition in the region, both THg and MeHg concentrations were low at all sites owing to relatively low groundwater DOC concentrations, as in many riparian zone soils in glaciated settings. This suggests that Hg contamination in riparian zones is unlikely to be a major concern in many riparian zones in glaciated settings, unless the riparian zone is a wetland with substantial organic matter or peat deposits, as peat is often associated with higher than normal Hg concentrations.7,13 Hydrological and Hg concentration data nevertheless indicate that the hydrogeomorphic setting of the riparian zone (i.e. topography, soil type, confining layer depth, subsurface drains), by regulating stream–riparian connectivity, determines the extent to which stream water Hg concentrations will influence riparian groundwater Hg (e.g. WR) or vice versa (e.g. LWD). For riparian zones located downstream from a potentially large source of Hg (e.g., concentrated urban development) in river valleys frequently subjected to flooding (e.g. WR), Hg concentrations are likely to be somewhat higher than in other, less-connected riparian hydrogeomorphic types. Nonetheless, although hydrogeomorphic classifications of riparian zones have shown to be useful to generalize riparian function with respect to nitrogen in glaciated settings,25–28 differences in riparian hydrogeomorphic characteristics in glaciated settings of the US Midwest do not appear to be associated with strong enough differences in ORP, SO42− and DOC values from one site to the next to generate clear differences in groundwater MeHg concentrations or %MeHg between sites. In places where past human activities (e.g. drainage) have depleted soil organic carbon (e.g. SSNS), less reduced oxidation–reduction conditions than expected based on site hydrology may affect soil biogeochemical conditions, and ultimately Hg cycling.Acknowledgements
This work was partially supported by USDA-NIFA grant # 2009-35112-05241 to P. Vidon, P. A. Jacinthe and M. E. Baker, and USGS-IWRRC grant # 06HQGR0084 to P. Vidon and C. Mitchell. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the USGS or USDA. Additional funding was also provided to P. Vidon by grants from the Indiana Academy of Science, and Indiana University-Purdue University, Indianapolis. Thanks are due to Mr Douglas Johnstone (WR site), Mr Jeffrey Phares (LWD site), and IndyParks, City of Indianapolis (SSNS site) for granting us access to the sites for the duration of the study.References
- D. Mergler, H. A. Anderson, L. H. M. Chan, K. R. Mahaffey, M. Murray, M. Sakamoto and A. H. Stern, Ambio, 2007, 36, 3–11 CrossRef CAS PubMed.
- A. M. Scheuhammer, M. W. Meyer, M. B. Sandheinrich and M. W. Murray, Ambio, 2007, 36, 12–18 CrossRef CAS PubMed.
- C. T. Driscoll, Y. J. Han, C. Y. Chen, D. C. Evers, K. F. Lambert, T. M. Holsen, N. C. Kamman and R. K. Munson, American Institute of Biological Sciences, 2007, vol. 57, pp. 17–28 Search PubMed.
- EPA, United States Environmental Protection Agency (USEPA), 2005/2006 National Listing of Fish Advisories, USEPA Fact Sheet EPA-823-F-07-003, USEPA, Washington, D.C., 2007, p. 7 Search PubMed.
- J. B. Shanley, N. C. Kamman, T. A. Clair and A. Chalmers, Ecotoxicology, 2005, 14, 125–134 CrossRef CAS PubMed.
- B. A. Branfireun and N. T. Roulet, Hydrol. Earth Syst. Sci., 2002, 6, 785–792 CrossRef.
- C. P. J. Mitchell, B. A. Branfireun and R. K. Kolka, Environ. Sci. Technol., 2008a, 42, 1010–1016 Search PubMed.
- K. A. Sinclair, Q. Xie and C. P. J. Mitchell, Environ. Pollut., 2012, 171, 207–215 CrossRef CAS PubMed.
- C. T. Driscoll, V. Blette, C. Yan, C. L. Schofield, R. Munson and J. Holapple, Water, Air, Soil Pollut., 1995, 80, 499–508 CrossRef CAS.
- S. G. Todorova, C. T. Driscoll, D. A. Matthews, S. W. Effler, M. E. Hines and E. A. Henry, Environ. Sci. Technol., 2009, 43, 6572–6778 CrossRef CAS PubMed.
- V. L. St. Louis, J. W. M. Rudd, C. A. Kelly, K. G. Beaty, R. J. Flett and N. Roulet, Environ. Sci. Technol., 1996, 30, 2719–2729 CrossRef CAS.
- J. M. Benoit, C. C. Gilmour, A. Heyes, R. P. Mason and C. Miller, American Chemical Society Symposium Series 835, Washington, DC, 2003 Search PubMed.
- C. P. J. Mitchell, B. A. Branfireun and R. K. Kolka, Appl. Geochem., 2008b, 23, 503–518 Search PubMed.
- P. Vidon, C. Allan, D. Burns, T. P. Duval, N. Gurwick, S. Inamdar, R. Lowrance, J. Okay, D. Scott and S. Sebestyen, J. Am. Water Resour. Assoc., 2010, 46, 278–298 CrossRef CAS.
- Y. H. Lee, K. H. Bishop and J. Munthe, Sci. Total Environ., 2000, 260, 11–20 CrossRef CAS PubMed.
- P. Selvendiran, C. T. Driscoll, J. T. Bushey and M. R. Montesdeoca, Environ. Pollut., 2008, 154, 46–55 CrossRef CAS PubMed.
- C. Hatcher and G. Filippelli, Water, Air, Soil Pollut., 2011, 219, 251–261 CrossRef CAS.
- T. P. Burt, Hydrol. Processes, 2005, 19, 2087–2089 CrossRef.
- R. J. Naiman, H. Decamps and M. E. McClain, Riparian: ecology, conservation, and management of streamside communities, Elsevier Academic Press, London, 2005, p. 430 Search PubMed.
- L. O. Hedin, J. von Fischer, N. E. Ostrum, B. P. Kennedy, M. G. Brown and G. P. Robertson, Ecology, 1998, 79, 684–703 Search PubMed.
- P. A. Jacinthe, P. M. Groffman, A. J. Gold and A. Mosier, J. Environ. Qual., 1998, 27, 156–164 CrossRef CAS.
- J. C. Clement, G. Pinay and P. Marmonier, J. Environ. Qual., 2002, 31, 1025–1037 CrossRef CAS PubMed.
- P. Vidon and A. R. Hill, Biogeochemistry, 2004, 71, 259–283 CrossRef CAS.
- K. J. Devito and A. R. Hill, Hydrol. Processes, 1997, 11, 485–500 CrossRef.
- R. Lowrance, L. S. Altier, J. D. Newbold, R. R. Schnabel, P. M. Groff-man, J. M. Denver, D. L. Correll, J. W. Gilliam, J. L. Robinson, R. B. Brinsfield, K. W. Staver, W. Lucas and A. H. Todd, Environ. Manage., 1997, 21, 687–712 CrossRef PubMed.
- A. R. Hill, in, Streams and Ground Waters, ed. J. B. Jones and P. J. Mulholland, Academic Press, San Diego, California, 2000, pp. 83–110 Search PubMed.
- A. J. Gold, M. Stolt, A. E. Rosenblatt, P. M. Groffman, K. Addy and D. Q. Kellogg, J. Am. Water Resour. Assoc., 2001, 37, 1457–1464 CrossRef CAS.
- P. Vidon and A. R. Hill, J. Am. Water Resour. Assoc., 2006, 42, 1099–1112 CrossRef CAS.
- NADP, National Atmospheric Deposition Program 2011 Annual Summary, NADP Data Report 2012-01, Illinois State Water Survey, University of Illinois at Urbana-Champaign, IL, 2012.
- NOAA, Climatological Data, Indianapolis, National Oceanic and Atmospheric Administration, National Climatic Data Center, Washington, DC, 2005 Search PubMed.
- Climatology of the United States, Monthly Station Normals of Temperature, Precipitation, and Heating and Cooling Degree Days 1971-2000: Indiana, Published by the US Department of Commerce, National Oceanic and Atmospheric Administration, National Climatic Data Center Washington, DC, 2002, p. 81.
- P. Vidon and P. E. Cuadra, Hydrol. Processes, 2010, 24, 1821–1833 CrossRef.
- P. Vidon and A. P. Smith, J. Am. Water Resour. Assoc., 2007, 43, 1524–1539 CrossRef.
- P. Vidon and A. P. Smith, Ecol. Restor., 2008, 26, 36–46 Search PubMed.
- L. L. Sanders, A Manual of Field Hydrology, Upper Saddle River, NJ, Prentice-Hall, 1998 Search PubMed.
- P. Vidon and A. R. Hill, Water Resour. Res., 2004, 40, W03201 CrossRef.
- L. S. Clesceri, A. E. Greenberg and A. D. Eaton, Standard methods for the examination of water and waste water, American Public Health Association, Washington, DC, 1998 Search PubMed.
- H. Hintelmann and R. D. Evans, Fresenius' J. Anal. Chem., 1997, 358, 378–385 CrossRef CAS.
- M. Horvat, L. Liang and N. S. Bloom, Anal. Chim. Acta, 1993, 282, 153–168 CrossRef CAS.
- R. W. Stoor, J. P. Hurley, C. L. Babiarz and D. E. Armstrong, Sci. Total Environ., 2006, 368, 99–110 CrossRef CAS PubMed.
- S. P. Rook, M.S. Thesis, SUNY College of Environmental Science and Forestry, Syracuse, New York, 2012.
- P. Vidon, L. E. Wagner and E. Soyeux, Biogeochemistry, 2008, 88, 257–270 CrossRef CAS.
- L. E. Wagner, P. Vidon, L. E. Tedesco and M. Gray, J. Hydrol., 2008, 362, 177–190 CrossRef.
- P. Vidon, Hydrol. Processes, 2012, 26, 3207–3215 CrossRef CAS.
- N. T. Baker, W. W. Stone, J. T. Wilson and M. T. Meyer, Occurrence and transport of agricultural chemicals in Leary Weber Ditch Basin, Hancock County, Indiana, 2003-04, U.S. Geological Survey Scientific Investigations Report 2006-5251, 2006.
- W. W. Stone and J. T. Wilson, J. Environ. Qual., 2006, 35, 1825–1835 CrossRef CAS PubMed.
- P. Vidon, H. Hubbard, P. Cuadra and M. Hennessy, Water, 2012, 4, 90–111 Search PubMed.
- C. L. Babiarz, J. M. Benoit, M. M. Shafer, A. W. Andren, J. P. Hurley and D. A. Webb, Biogeochemistry, 1998, 41, 237–257 CrossRef CAS.
- A. A. Szogi, P. G. Hunt, E. J. Sadler and D. E. Evans, Appl. Eng. Agr., 2004, 20, 189–200 CrossRef.
- W. J. Mitsch and J. G. Gosselink, Wetlands. New York, John Wiley and Sons, Inc., 2000, p. 920 Search PubMed.
- P. Vidon, P. A. Jacinthe, X. Liu, K. Fisher and M. Baker, J. Am. Water Resour. Assoc. Search PubMed , in press.
| This journal is © The Royal Society of Chemistry 2013 |
