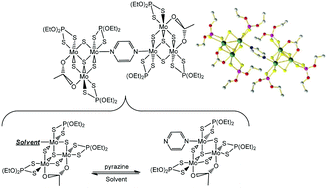
New triangular Mo(IV) and W(IV) incomplete cuboidal cluster complexes containing three η2-diethyldithiophosphates (dtp, one per metal centre) and a η2(μ)-AcO or η2(μ)-dtp ligand bridging two of these centres have been prepared as mono-substituted derivatives and characterized by the usual techniques plus single crystal X-ray diffraction (XRD) analysis in some cases. In the compounds, the single substitutionally available unlocked position is coordinated to different N-donor ligands (1,2-bis(pyridyl)ethylene, pyrimidine, pyrazine) or to the residual solvent. The compounds are very labile and the N-donor ligands are only weakly coordinated, being easily substituted by other donors or coordinating solvents such as acetonitrile. This lability has been explored kinetico-mechanistically by stopped-flow techniques at varying temperatures for the compound [Mo3S4(η2-dtp)3(η2(μ)-dtp)(H2O)]. By doing so, the nature of the activation process leading to substitution reactions on these complexes has been established as associatively activated and dominated by a very low formation equilibrium constant, that is, by the substitution of the incoming N-donor ligand. Furthermore, the existence of a non-dissociatively activated trigonal 60° fluxional twist has also been established for the η2-dtp ligand on the single unlocked position. The lability of the system has also enabled the association in dimers of the acetato derivative, i.e. [Mo3S4(η2-dtp)3(η2(μ)-AcO)(H2O)], via a pyrazine bridging ligand. After a very careful tuning of the reaction conditions, as established from the kinetico-mechanistic studies, the first single-bridged unsupported dimeric association of {Mo3S4} units, i.e. [{Mo3S4(η2-dtp)3(η2(μ)-AcO)}2(μ-pyrazine)], has been structurally characterised.

 Please wait while we load your content...
Please wait while we load your content...