Chiral vanadium(v) complexes with 2-aminoglucose Schiff-base ligands and their solution configurations: synthesis, structures, and DFT calculations†
Abstract
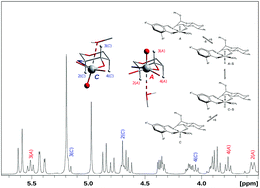
The sugar-modified Schiff-base ligands derived from benzyl 2-deoxy-2-salicylideneamino-α-D-glucopyranoside (H2L5-Br and H2L3-OMe) were used to prepare the chiral oxidovanadium(V) complexes [VO(L5-Br)(OMe)] (1) and [VO(L3-OMe)(OMe)] (2) which can be isolated from a methanol solution as the six-coordinate complexes with an additional methanol ligand [VO(L5-Br)(OMe)(MeOH)] (1–MeOH) and [VO(L3-OMe)(OMe) (MeOH)] (2–MeOH). Both complexes crystallize in the orthorhombic space group P212121 together with two solvent molecules of methanol as 1–MeOH·2MeOH and 2–MeOH·2MeOH. In both crystal structures, only diastereomers with A configuration at the chiral vanadium centre (OC-6-24-A) are observed which corresponds to an cis configuration of the oxido group at the vanadium centre and the benzyl group at the anomeric carbon of the sugar backbone. Upon recrystallization of 2–MeOH from chloroform, the five-coordinate complex 2 was obtained which crystallizes in the monoclinic space group P21 with one co-crystallized chloroform molecule (2·CHCl3). For the chiral vanadium centre in 2·CHCl3, a C configuration (SPY-5-43-C) is observed which corresponds to an trans structure as far as the orientations of the oxido and benzyl groups are concerned. 1H and 51V NMR spectra of 1 and 2 indicate the presence of two diastereomers in solution. Their absolute configurations can be assigned based on the magnetic anisotropy effect of the oxidovanadium group. This effect leads to significant differences for the 1H NMR chemical shifts of the H-2 (1.1 ppm) and H-3 protons (0.3 ppm) of the glucose backbone of the two diastereomers, with the downfield shift observed for the H-2 proton of the C-configured and the H-3 proton of the A-configured diastereomer at the vanadium centre. For 1 and 2 the difference between the 51V NMR chemical shifts of the two diastereomers is 30 and 28 ppm, respectively. Also in the 13C NMR significant chemical shift differences between the two diastereomers are observed for the carbon atoms C2 (2 ppm) and C3 (4 ppm). DFT calculations of the NMR chemical shift parameters have been performed which are in good agreement with the experimental data. Moreover, the isomerization mechanism between the diastereomers is analysed on the basis of DFT calculations which indicate the required presence of methanol molecules as protic donors.
- This article is part of the themed collection: Vanadium in Inorganic Chemistry

 Please wait while we load your content...
Please wait while we load your content...