Vanadium(iv/v)–p-dioxolene temperature induced electron transfer associated with ligation/deligation of solvent molecules†
Abstract
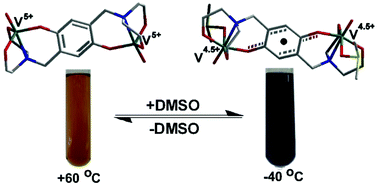
Reaction of an aqueous solution of NaVO3 or a methanol solution of [VO(acac)2] with
- This article is part of the themed collection: Vanadium in Inorganic Chemistry

 Please wait while we load your content...
Please wait while we load your content...