Discrete spherical hexadecavanadates incorporating a bromide with oxidative bromination activity†
Abstract
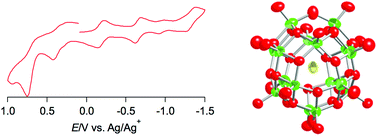
Two discrete hexadecavanadates, (n-Bu4N)4[V16O38(X)] (X = Cl− (1) and X = Br− (2)), were synthesized by a reaction of [V10O26]4− with a template anion resulting in the incorporation of chloride or bromide in the {V16} spherical cluster framework. The reaction of [V10O26]4− with
- This article is part of the themed collection: Vanadium in Inorganic Chemistry

 Please wait while we load your content...
Please wait while we load your content...