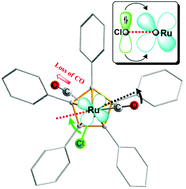
The formation of an active 16-electron ruthenium sec-alkoxide complex via loss of the CO ligand is an important step in the mechanism of the racemization of sec-alcohols by (η5-Ph5C5)Ru(CO)2X ruthenium complexes with X = Cl and OtBu. Here we show with accurate DFT calculations the potential energy profile of the CO dissociation pathway for a series of relevant (η5-Ph5C5)Ru(CO)2X complexes, where X = Cl, OtBu, H and COOtBu. We have found that the CO dissociation energy increases in the following order: OtBu (lowest), Cl, COOtBu and H (highest). Using the distance between ruthenium and CCO, r = Ru–CCO, as a constraint, and by optimizing all other degrees of freedom for a range of Ru–CO distances, we obtained relative energies, ΔE(r) and geometries of a sufficient number of transient structures with the elongated Ru–CO bond up to r = 3.4 Å. Our calculations provide a quantitative understanding of the CO ligand dissociation in (η5-Ph5C5)Ru(CO)2Cl and (η5-Ph5C5)Ru(CO)2(OtBu) complexes, which is relevant to the mechanism of their catalytic activity in the racemization of alcohols. We recently reported that exchange of the CO ligand by isotopically labeled 13CO in the Ru–OtBu complex occurs twenty times faster than that in the Ru–Cl complex. This corresponds to a difference of 1.8 kcal mol−1 in the CO dissociation energy (at room temperature). This is in very good agreement with the calculated difference between the two potential energy curves for Ru–OtBu and Ru–Cl complexes, which is about 1.8–2 kcal mol−1 around the corresponding transition states of the CO dissociation. The calculated difference in the total energy for CO dissociation in (η5-Ph5C5)Ru(CO)2X complexes is related to the stabilization provided by the X group in the final 16-electron complexes, which are formed via product-like transition states. In addition to the calculated transition states of CO dissociation in Ru–OtBu and Ru–Cl complexes, the calculated transient structures with the elongated Ru–CO bond provide insight into how the geometry of the ruthenium complex with a potent heteroatom donor group (X) gradually changes when one of the COs is dissociating.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...