 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sorbitol dehydration into isosorbide in a molten salt hydrate medium
Jianrong Li, Antonio Spina, Jacob A. Moulijn and Michiel Makkee*
Catalysis Engineering, ChemE, Delft University of Technology, Julianalaan 136, 2628 BL, Delft, The Netherlands. E-mail: m.makkee@tudelft.nl; Fax: +31 15 278 5006; Tel: +31 15 278 1391
First published on 6th March 2013
Abstract
The sorbitol conversion in a molten salt hydrate medium (ZnCl2; 70 wt% in water) was studied. Dehydration is the main reaction, initially 1,4- and 3,6-anhydrosorbitol are the main products that are subsequently converted into isosorbide; two other anhydrohexitols, (1,5- and 2,5-), formed are in less amounts and do not undergo further dehydration. Besides dehydration, depending on the temperature, sorbitol was partly epimerized into galactitol, which was further converted to anhydrohexitols or di-anhydrohexitols (mainly isoidide). Epimerization of galactitol into sorbitol was not observed. Temperature (150 to 220 °C) is a crucial factor; at low temperature the reaction rate is low but the selectivity is high, at elevated temperature (over 200 °C) the rate is high but extensive amounts of byproducts were produced. At the optimal temperature range, without using any co-catalysts, full conversion is achieved, and the isosorbide percent yield is above 85% on a molar basis; (1,5- and 2,5-) anhydrohexitols are the major byproducts. The sorbitol conversion reaction pathway has been investigated.
Introduction
Conversion of biomass is attracting a lot of attention from the academic and the industrial fields.1,2 Lignocellulosic biomass, so-called 2nd generation biomass, is abundantly present and in many cases it does not compete with the food chain. Lignocellulosic biomass is refractory towards moderate (temperature) chemical conversion. Previously we have reported a general route for converting cellulose to isosorbide in a molten salt hydrate medium (ZnCl2 70 wt% in water).3 In this type of medium, accessibility of cellulose is good and further dissolution and depolymerization/hydrolysis of cellulose take place at relatively high rates. When lignocellulosic biomass is used as feedstock, the molten salt hydrate medium also works well. It has been demonstrated that sequential hydrolysis, hydrogenation and dehydration reactions in the medium (“in situ”) are accomplished, resulting in relatively easy separation of isosorbide, because of the reduced polarity of the product.4Through simultaneous hydrolysis and hydrogenation, we are able to obtain full conversion of cellulose, and essentially >95% selectivity to sorbitol is achieved in the molten salt hydrate medium.5 Further dehydration of sorbitol into sorbitan and isosorbide can take place in the same medium, as shown in Fig. 1.
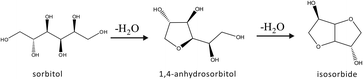 | ||
| Fig. 1 Dehydration of sorbitol to 1,4-anhydrosorbitol and, subsequently, to isosorbide (the scheme is similar when the intermediate 3,6-anhydrosorbitol is applied). | ||
Three 1,4:3,6-dianhydrohexitol (isosorbide, isomannide, and isoidide) structures exist,6 as shown in Fig. 2.
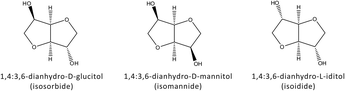 | ||
| Fig. 2 Structures of 1,4:3,6-dianhydrohexitols. | ||
1,4:3,6-Dianhydrohexitols have attracted a lot of attention, especially isosorbide. Isosorbide is an attractive platform molecule; it is a sustainable diol with a melting temperature of 61–62 °C and high thermal stability of up to 270 °C. Isosorbide and its derivatives possess high potential values in industrial applications, such as application in medicine, in synthetic chemistry,7–11 as sustainable solvents;12 as building blocks for poly-esters.13 Reviews on the use of 1,4:3,6-dianhydrohexitols in poly-esters and polymers have been published by Braun and Bergman,14 Kricheldorf,15 and Fenouillot et al.16
Dehydration of hexitols can be realized by both homogeneous catalysis, for example, in strong mineral acid media (hydrochloride acid,17 sulfuric acid18,19) and heterogeneous catalysis, for instance, SiO2–Al2O3,20 bimetallic catalysts,21 and H3PW12O40/SiO2.22 Without using any acid catalyst, sorbitol dehydration in high temperature liquid water was recently reported by Yamaguchi et al.23
A selection of literature on catalytic dehydration of sorbitol (hexitol) can be found in Table 1. The dehydration of hexoses is more cumbersome than that of hexitols, since the reactivity of hexoses is higher and they are more prone to degradation. A good overview of the dehydration of hexoses has been provided by Zakrzewska et al.24
| Phases formed by catalysts and reactants | Catalysts | Conditions | Conversion (%) | Yield (%) | Authors/remarks | ||
|---|---|---|---|---|---|---|---|
| (1,4+3,6-)Mono-anhydrosorbitol | Other mono-anhydrohexitols | Isosorbide | |||||
| n.r., Not reported.a Yields = product/initial sorbitol × 100% (mol mol−1).b 1,4-Anhydro-D-glucitol.c 1,4-Sorbitan.d 1,4-Anhydro-L-glucitol (13%) + 2,5-anhydro-L-iditol (2%).e 1,4-Anhydro-D-glucitol (74%) + 3,6-anhydro-D-glucitol (0%).f 2,5-Anhydro-L-iditol (6%) + 2,5-anhydro-D-mannitol (1%).g 1,4-Anhydro-D-glucitol (15.6%) + 3,6-anhydro-D-glucitol (5.6%).h 1,5-Anhydro-D-glucitol (4.5%) + 2,6-anhydro-D-glucitol (1.6%) + 2,5-anhydro-L-iditol (6.0%) + 2,5-anhydro-D-mannitol (2.2%).i 1,4-Anhydro-D-glucitol (0.8%) + 3,6-anhydro-D-glucitol (1.7%).j 1,5-Anhydro-D-glucitol (3.4%) + 2,6-anhydro-D-glucitol (1.4%) + 2,5-anhydro-L-iditol (3.0%) + 2,5-anhydro-D-mannitol (3.6%). | |||||||
| I. Liquid (water as solvent for both catalysts and reactants) | 2 N HCl | 100 °C, time not reported | 100 | 85b | 15d | 0 | Barker17 |
| 3 M H2SO4 | 104 °C, 28 h | 96 | 74e | 7f | 15 | Bock et al.18 | |
| 1% H2SO4 | 135 °C, 20 h | 100 | 8 | n.r. | 77 | Fleche and Huchette19 | |
| 1% H3PO4 | 87 | 68 | n.r. | 12 | |||
| 1% HCl | 100 | 12 | n.r. | 76 | |||
| 1% SnCl4 | 76 | 64 | n.r. | 7 | |||
| 1% AlCl3 | 96 | 79 | n.r. | 8 | |||
| ZnCl2 hydrate (CuCl2 as a co-catalyst) | 180 °C, 1.5 h, 4 MPa H2 | 99 | 4a | 95a | Menegassi de Almeida et al.3 | ||
| II. Catalyst solid, reactants molten (sorbitol melting point is 95 °C, water formed by dehydration) | 3 Å Zeolites | 270 °C, 2 h | 80 | 21g | 14h | 29 | Kurszewska et al.26 |
| 290 °C, 3 h | n.r. | 2i | 11j | 81 | |||
| Anhydrous pyridinium chloride | 120 °C, 10 h | n.r. | 30b | n.r. | 0 | Duclos et al.27 Pyridinium chloride Mp = 140–146 °C | |
| 140 °C, 10 h | n.r. | 60b | n.r. | 8 | |||
| 160 °C, 10 h | n.r. | 47b | n.r. | 32 | |||
| III. Catalyst solid, reactants in aqueous | SiO2–Al2O3 | 245 °C, 24 h, 3 MPa He | 94 | 20c | n.r. | 62 | Li and Huber20 |
| Sn(IV) phosphate | 300 °C, LHSV = 0.24 h−1 | 72 | 24c | n.r. | 47 | Gu28 Fixed-bed reactor | |
| Zr(IV) phosphate | 56 | 14c | n.r. | 29 | |||
| Ti(IV) phosphate | 97 | 3c | n.r. | 45 | |||
| Sulfated copper oxide 300 | 200 °C, 4 h (TOS) | 100 | n.r. | n.r. | 58 | Xia29 Fixed-bed reactor In sulfated copper oxide 300, 300 means the catalyst calcined at 300 °C. Below are the same. | |
| Sulfated copper oxide 400 | 100 | n.r. | n.r. | 61 | |||
| Sulfated copper oxide 500 | 100 | n.r. | n.r. | 50 | |||
| Sulfated copper oxide 600 | 100 | n.r. | n.r. | 56 | |||
| Sulfated copper oxide 650 | 100 | n.r. | n.r. | 67 | |||
| Sulfated copper oxide 750 | 64 | n.r. | n.r. | 3 | |||
| Sulfated nickel oxide 600 | 75 | n.r. | n.r. | 16 | |||
| Sulfated aluminum oxide 600 | 97 | n.r. | n.r. | 58 | |||
| Amberlite C200 | 135 °C, 20 h | 97 | 29 | n.r. | 55 | Fleche19 | |
| Cu–Pt | 220 °C, 2.5 h, 4 MPa H2 | 94 | 73 | 21 | Montassier21 | ||
| RANEY® copper | |||||||
| H2PtCl6 as a metallic modifier precursor | |||||||
| Pt/Cu = 100 (initial surface Cu, atomic%) | |||||||
Table 1 can be divided into three groups according to the phases of catalysts and reactants. It is generally agreed that sorbitol dehydration into isosorbide takes place through the intermediates 1,4- and 3,6-mono-anhydrosorbitols, whereas the commonly observed isomers 1,5- and 2,5-mono-anhydrohexitols are stable under the reaction conditions.
The classic way of catalytic dehydration of sorbitol is based on aqueous mineral acid catalysts, as shown in the first group in Table 1. Among the strong mineral acids (H2SO4, H3PO4, HCl), H2SO4 seems to be the best under the same conditions (135 °C, 20 h). Through mechanistic studies, Fleche and Huchette19 and Dosen-Micovic and Cekovic25 suggested an SN2 mechanism for acid-catalyzed cyclodehydration reaction of hexitols. Lewis acids (SnCl4, AlCl3) mainly lead to the 1,4-monoanhydrosorbitol and are not efficient for the second step of dehydration to produce isosorbide. To avoid the drawbacks of using strong mineral acids, i.e. environmental problems, corrosion, etc., some researchers focus on the study of using solid acid catalysts such as H-zeolites (3 Å zeolites), ion-exchange resins (Amberlyst), anhydrous pyridinium chloride, and bimetallic catalysts (Cu–Pt, RANEY® copper, acid promoted Pt), as shown in the second and the third groups in Table 1. When solid acid catalysts are used, the fed sorbitol can be solid (second group, sorbitol melting point is 95 °C) or in an aqueous solution (third group). The main advantage of solid acid catalysts is the easy product/catalyst separation. However, most solid acid catalysts exhibit a low activity resulting in long reaction times and/or high reaction temperatures. In addition, the yields are limited.
Due to the low activity and poor selectivity of classic solid acid catalysts, the development of more efficient solid acid catalysts has been tried, for instance, metal sulfates and phosphates because of their large number of moderate strength acidic sites.
From Table 1, it is clear that solid acid catalysts generally do not result in satisfactory yields of isosorbide.
Compared to concentrated strong mineral acids some molten salt hydrates are more attractive, because they are more active and cause less corrosion. Our previous work3 briefly introduced the strategy of converting cellulose into isosorbide using molten ZnCl2 hydrate as the solvent and catalyst. In this paper we will report in detail the step of converting sorbitol into isosorbide by dehydration reactions. It was found in our previous work3,4 that CuCl2 or NiCl2 as a co-catalyst increased the dehydration activity and the isosorbide selectivity. However, considering our whole process, especially the solvent recycle, avoiding of co-catalysts is preferred. Therefore, in this paper, we will discuss the sorbitol dehydration reaction in a molten ZnCl2 hydrate medium without using any co-catalysts.
The objective of this work is to study the selective dehydration of sorbitol into isosorbide in a molten salt hydrate medium, to investigate the reaction pathway and to study and optimize the process to obtain as high isosorbide yield as possible.
Experimental
Materials
Zinc chloride (≥97%), D-sorbitol (≥99.5%), D-mannitol, D-talitol, D-allitol, L-iditol, D-galactitol (≥99%), 1,5-anhydrosorbitol, 1,5-anhydromannitol, 2,5-anhydromannitol, isosorbide (≥98%) and isomannide (≥95%) were purchased from Sigma-Aldrich.Methods
A transparent 70 wt% ZnCl2 in water solution was prepared prior to use. Sorbitol was added to the solution (the weight ratio of sorbitol to ZnCl2 solution is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12), under continuous stirring until the system became a transparent solution again and was added to the reactor.
12), under continuous stirring until the system became a transparent solution again and was added to the reactor.Sorbitol dehydration experiments were carried out in a batch titanium autoclave (Premex). The batch reactor, equipped with a magnetic stirrer, has a volume of 500 mL.
After adding sorbitol solution the reactor was flushed and pressurized until 30 bar with N2, then stirring (1500 rpm), and heating was switched on. This time was assigned as t = 0. Depending on the set temperature (range of 150 to 220 °C was studied), it took 30 to 60 minutes to reach the reaction temperature.
Samples were taken at selected reaction times. They were diluted with water and solid products, if present, were separated by filtration. The liquid phase was analyzed by a HPLC, equipped with a Refractive Index (RI) detector and a Phenomenex Rezex RCM-monosaccharide column (8%; Ca2+, 300 × 7.80 mm) at 80 °C with degassed demi water as eluent.
An example of a HPLC pattern used for calibration of chemicals involved is shown in Fig. 3. The peaks (ZnCl2, sorbitol, 2,5-anhydromannitol, 1,5-anhydrosorbitol, 1,4- and 3,6-anhydrosorbitol, isosorbide, galactitol) in the figure correspond to reactants and products present in the system (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 180 °C, 240 min). The other lines are based on separate injection of 1,5-anhydromannitol, isomannide, D-allitol, D-talitol, D-mannitol, and L-iditol. The conversion and selectivity were determined by individual calibrations and the mass balance was closed by quantifying unknowns with an average response factor.
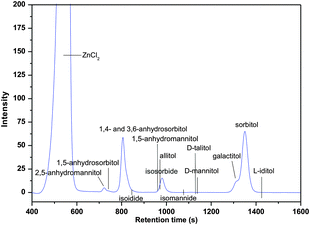 | ||
| Fig. 3 Relative positions of hexitols, monoanhydrohexitols and dianhydrohexitols in HPLC analysis (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 180 °C, 240 min). | ||
Results
Typical HPLC analysis results
A typical set of HPLC patterns of sorbitol conversion in ZnCl2 molten salt hydrate medium (at 180 °C) is given in Fig. 4.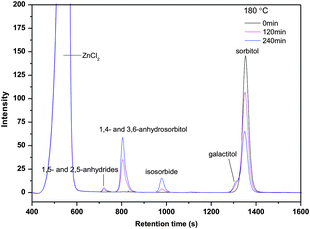 | ||
| Fig. 4 Typical HPLC pattern of sorbitol conversion in ZnCl2 molten salt hydrate medium (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 180 °C). | ||
The results show that the sorbitol peak decreases with prolonged reaction time. Sorbitol is mainly converted into 1,4- and 3,6-anhydrosorbitols, and a small amount of 1,5- and 2,5-anhydrides (from HPLC calibration results, they are assigned as 1,5-anhydrosorbitol and 2,5-anhydromannitol, see Fig. 3) is detected. Some isosorbides have been formed through dehydration of 1,4- and 3,6-anhydrosorbitols. Traces of galactitol are observed; the amounts increase with time.
Product distribution as a function of reaction time and temperature
In sugar chemistry the selectivity is more important than the activity because the molecules are very reactive and prone to degradation reactions. Therefore, a careful analysis was made from the product chromatograms at different temperatures. Fig. 5–7 give a selection of the results.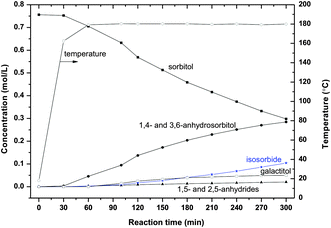 | ||
| Fig. 5 Sorbitol conversion in ZnCl2 molten salt hydrate medium (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 180 °C). | ||
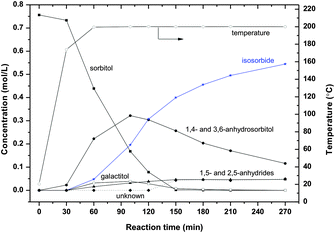 | ||
| Fig. 6 Sorbitol conversion in ZnCl2 molten salt hydrate medium (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 200 °C). | ||
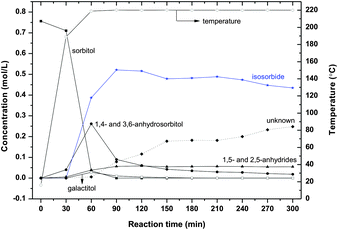 | ||
| Fig. 7 Sorbitol conversion in ZnCl2 molten salt hydrate medium (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 220 °C). | ||
At 180 °C the main products are 1,4- and 3,6-anhydrosorbitols and small amounts of 1,5- and 2,5-anhydrides are visible; the yield of isosorbide is clearly noticeable, see Fig. 5. It is striking that the amount of 1,5- and 2,5-anhydrides shows a plateau, less than 3% is formed after 300 minutes reaction. Small amounts of galactitol up to 6% were detected.
At 200 °C isosorbide is the main product and 1,4- and 3,6-anhydrosorbitols show a maximum. Increasing (small) amounts of 1,5- and 2,5-anhydrides are visible, see Fig. 6. From a molar balance it can be calculated that after 270 minutes of reaction a small amount of “unknown” has been formed (around 6% yield). With prolonged reaction time (not shown in the figure) for which the 1,4- and 3,6-anhydrosorbitols were completely converted, the isosorbide yield is over 85% on a molar basis. The galactitol yield as a function of time on stream reaching a maximum of up to 5% was observed.
In order to find the optimal temperature and to get a good understanding of the degradation reactions, experiments were performed at higher temperature. At 220 °C (Fig. 7) isosorbide is formed at a high rate, after 90 minutes the yield of isosorbide is 69%. At longer reaction times the isosorbide yield decreases and increasing amounts of unknown products are formed, 33% yield after 300 minutes reaction, while the isosorbide yield decreases to 58%.
From Fig. 5–7, it is clear that the sorbitol conversion rate increases with increasing temperature. Below 180 °C, the reaction is very slow; above 180 °C the reaction is feasible for a practical process. At 200 °C, after 120 minutes reaction, about 90% sorbitol has been converted. At 220 °C, after 60 minutes essentially full sorbitol conversion is obtained.
In the product patterns galactitol is observed, see for instance Fig. 5. Fig. 8 shows the yields of galactitol for different temperatures. The maximum in the curves strongly suggests a kinetic scheme analogous to the sorbitol reaction scheme.
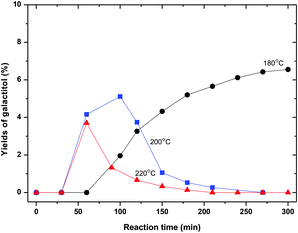 | ||
| Fig. 8 Galactitol yield as a function of temperature (70 wt% ZnCl2 600 g, sorbitol 50 g). | ||
Fig. 9 shows yields representing “unknown” products, calculated from the molar balances based on the HPLC diagrams. The amounts strongly increase with increasing temperature. Below 200 °C the amounts formed are negligible. At 220 °C the yields are dramatic, suggesting extensive degradation.
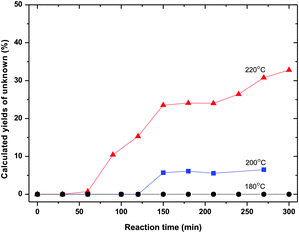 | ||
| Fig. 9 “Unknown” yield as a function of temperature (70 wt% ZnCl2 600 g, sorbitol 50 g). | ||
Fig. 10 shows the relationship of the product selectivity versus sorbitol conversion at varying temperatures. Each data point refers to a different reaction time. The main reactions are (i) the formation of 1,4 and 3,6-anhydrosorbitols reacting further in high yield with isosorbide and (ii) the formation of 1,5- and 2,5-anhydrides not undergoing further dehydration. At full sorbitol conversion the isosorbide yields upon further conversion can increase (Fig. 10b, 200 °C) or decrease by dominating degradation reactions (Fig. 10c, 220 °C).
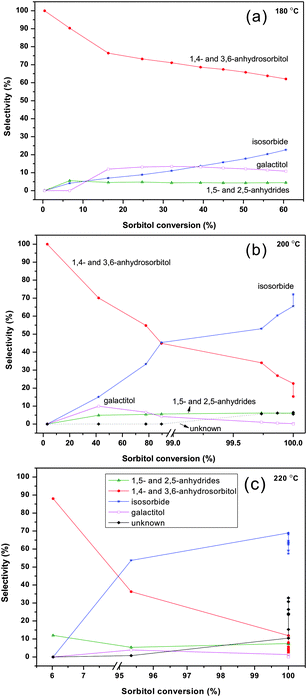 | ||
| Fig. 10 Product selectivity versus sorbitol conversion at varying temperatures (in ZnCl2 molten salt hydrate medium, 70 wt% ZnCl2 600 g, sorbitol 50 g; (a) 180 °C; (b) 200 °C; (c) 220 °C). The data correspond to those presented in Fig. 5–7; and the reaction time does vary. | ||
Discussion
The present data concern the ZnCl2 hydrate without any additives. Apart from technical reasons (convenient in a practical process) the absence of additives greatly facilitates the interpretation of the results. 200 °C seems to be an optimal temperature where the maximal percent yield of isosorbide is >85% on a molar basis (at optimal reaction time). At higher temperature the degradation reactions play an increasing role. The yield found in the present study is lower than that reported in our earlier work.3 It should be noted that the conditions are different. In the earlier work a high hydrogen pressure was applied and the CuCl2 co-catalyst was present. Because of the value of the main by-products, 1,5- and 2,5-anhydrides, and the small amount of “unknown” formed at 200 °C of this study, at this stage for our system further optimization based on conversion of the mono-anhydrohexitols is not the highest priority.Reaction pathway
The product distributions suggest a rather simple pathway. ZnCl2 hydrate medium (70 wt% in water) has a pH of around 1–2. From the literature it is known that acids such as sulphuric acid catalyze this reaction by protonation with an SN2 mechanism.19,30 However, in a ZnCl2–H2O catalyzed system, the mechanism is different from a mineral acid catalyzed system. In the catalytic reaction a complex is involved of sorbitol and ZnCl2–H2O.31 In the first dehydration step two types of monoanhydrosorbitols are formed, the first type (1,4- and 3,6-anhydrosorbitol) reacts sequentially to dianhydrosorbitol (the concentration of these monoanhydrosorbitols goes through a maximum) and the second type (1,5- and 2,5-anhydrides) is stable towards further dehydration (after a period of increasing concentration, a constant concentration level is observed). This observation is corroborated by the molecular modeling study on the sorbitol dehydration reactions.31In the reaction pathway sorbitol isomerization (epimerization) into galactitol is included (by a 1,4-ring closure and subsequent 1,4-ring opening). The opposite reaction of galactitol epimerization into sorbitol is, however, not significant. Galactitol and sorbitol are very similar and it is not surprising that they give the same types of products. Double dehydration of galactitol will mainly result in the formation of isoidide. Some experiments starting with galactitol as an initial reactant were performed. The results confirmed the above statements. Besides 1,4- and 3,6-anhydrogalactitols, relatively small amounts of other mono-anhydrohexitols are also formed but not indicated in the reaction pathway. However, since the maximum amount of galactitol observed in this study is only about 6%, in the deconvolution of the HPLC patterns the tiny amounts of products of galactitol were not explicitly accounted for.
Isomerization of isosorbide into isoidide and isomannide is also included in the reaction pathway, because we detected amounts of isoidide and isomannide at higher temperature reactions such as T ≥ 190 °C, a HPLC pattern is given in Fig. 11 as an example. However, these isomers are not assigned specifically in the evaluation of the different temperature reactions (Fig. 5–7). As shown in Fig. 11, the isoidide peak overlaps with 1,4- and 3,6-anhydrosorbitol peaks and was counted as 1,4- and 3,6-anhydrosorbitols when we calculated the concentration profiles. In a stability test of pure isosorbide in the reaction medium at 200 °C we found besides degradation also the formation of isoidide and isomannide, confirming the occurrence of isosorbide isomerization.
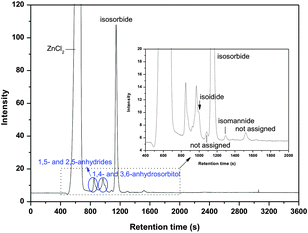 | ||
| Fig. 11 Sorbitol conversion in ZnCl2 molten salt hydrate medium (70 wt% ZnCl2 600 g, sorbitol 50 g, T = 200 °C, 270 min). | ||
Based on the observations, a summarized reaction pathway is proposed as below (Fig. 12).
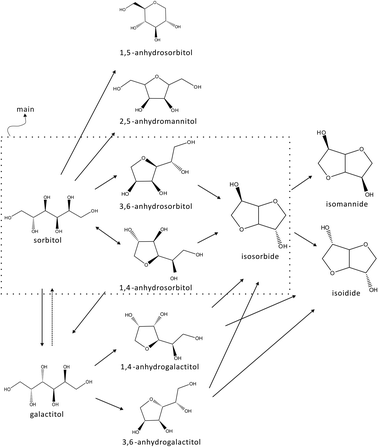 | ||
| Fig. 12 Reaction pathway of sorbitol conversion in ZnCl2 molten salt hydrate medium. | ||
“Unknown” elucidations/compositions
The amount of “unknown” was calculated by molar balance.Isosorbide yield decreases when a prolonged reaction time at high temperature (220 °C) was applied. It is reported that isosorbide can be isomerized into isoidide and isomannide: starting with isosorbide in water with a nickel catalyst promoted by Cu at 220 °C and 2 hours reaction, the system ends up with an equilibrium of 35.5% isosorbide, 58.9% isoidide, and 5.7% isomannide.32 In our experiments we observe significantly slower isomerisation, suggesting that compared with such a system ZnCl2 stabilizes isosorbide, inhibiting isomerization. As discussed in the Reaction pathway section, isoidide and isomannide were detected in experiments, when isosorbide is used as the reactant. However, the total amount of isoidide and isomannide (<4%) is less than the total isosorbide conversion. Thus, other reactions besides isomerization have to occur, the more so, because some isoidide will originate from the galactitol pathway. Moreover, isosorbide dehydration and perhaps coupled herewith degradation reactions occur. In our analysis some small not assigned peaks are observed (Fig. 11). These peaks are part of the “unknowns”. At present we are not able to assign these peaks to a chemical structure.
In our results, at 220 °C, from 90 to 300 minutes, isosorbide weight yield drops from 69% to 58%, “unknown” yield increases from 10% to 33%. Thus, isosorbide is involved in the degradation reactions, but it is not the only molecule which is responsible for the unknowns. Also the other products, the anhydrohexitols, exhibit degradation reactions.
We tried to analyze the degradation products. By repeated washing and filtering, certain amounts of black solid by-products were collected from the final residue of sorbitol conversion at 220 °C experiments, which proved that a certain amount of (probably) oxygenated carbonaceous by-products exists. Quantification of the amount of solids versus reaction time and temperature is under investigation.
It was decided not to go in further detailed analysis of the chemistry of the degradation reactions but to accept that the reaction temperature has to be low.
Conclusions
The selective dehydration of sorbitol into isosorbide using ZnCl2 (70 wt% in water) molten salt hydrate as reaction medium was studied; without using any co-catalysts, full conversion of sorbitol with high selectivity to isosorbide (>85% on molar basis) was achieved. The reaction pathway is that of a serial pathway. The 1,4- and 3,6-anhydrosorbitols are further dehydrated into isosorbide, the 1,5- and 2,5-anhydrides are not further dehydrated. Process conditions are optimized, depending on the reaction conditions, isomerization, epimerization and degradation can play a significant and decisive role. Temperature is a crucial factor for the reaction. At low temperature the reaction rate is low, at elevated temperature (over 200 °C) the rate is high but extensive amounts of byproducts are produced.Acknowledgements
Authors would like to thank BIOeCON and PETROBRAS for their financial support; Prof. Dr Ir Herman van Bekkum is acknowledged for his stimulating discussions.Notes and references
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS.
- R. Menegassi de Almeida, J. Li, C. Nederlof, P. O'Connor, M. Makkee and J. A. Moulijn, ChemSusChem, 2010, 3, 325–328 CrossRef CAS.
- R. Menegasse de Almeida, S. Daamen, J. A. Moulijn, M. Makkee and P. O'Connor, European Patent, 2009, EP2100972A1, Chemical Indexing Equivalent to 151:339509 (WO) Search PubMed.
- J. Li, H. S. M. P. Soares, J. A. Moulijn and M. Makkee, Catal. Sci. Technol. 10.1039/c3cy20808g.
- H. G. Fletcher, Jr. and R. M. Goepp, Jr., J. Am. Chem. Soc., 1946, 68, 939–941 CrossRef.
- D. Abenhaiem, A. Loupy, L. Munnier, R. Tamion, F. Marsais and G. Queguiner, Carbohydr. Res., 1994, 261, 255–266 CrossRef CAS.
- S. Chatti, M. Bortolussi and A. Loupy, Tetrahedron, 2000, 56, 5877–5883 CrossRef CAS.
- S. Kumar and U. Ramachandran, Tetrahedron, 2005, 61, 4141–4148 CrossRef CAS.
- Y. Zhu, M. Durand, V. Molinier and J.-M. Aubry, Green Chem., 2008, 10, 532–540 RSC.
- A. Loupy and D. A. Monteux, Tetrahedron, 2002, 58, 1541–1549 CrossRef CAS.
- P. Tundo, F. Arico, G. Gauthier, L. Rossi, A. E. Rosamilia, H. S. Bevinakatti, R. L. Sievert and C. P. Newman, ChemSusChem, 2010, 3, 566–570 CrossRef CAS.
- M. Okada and K. Aoi, Curr. Trends Polym. Sci., 2002, 7, 57–70 CAS.
- D. Braun and M. Bergmann, J. Prakt. Chem./Chem.-Ztg., 1992, 334, 298–310 CrossRef CAS.
- H. R. Kricheldorf, J. Macromol. Sci., Rev. Macromol. Chem. Phys., 1997, C37, 599–631 CrossRef CAS.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- R. Barker, J. Org. Chem., 1970, 35, 461–464 CrossRef CAS.
- K. Bock, C. Pedersen and H. Thoegersen, Acta Chem. Scand. Ser. B, 1981, B35, 441–449 CrossRef CAS.
- G. Fleche and M. Huchette, Starch/Staerke, 1986, 38, 26–30 CrossRef CAS.
- N. Li and G. W. Huber, J. Catal., 2010, 270, 48–59 CrossRef CAS.
- C. Montassier, J. C. Menezo, J. Naja, P. Granger, J. Barbier, P. Sarrazin and B. Didillon, J. Mol. Catal., 1994, 91, 119–128 CrossRef CAS.
- P. Sun, D. H. Yu, Y. Hu, Z. C. Tang, J. J. Xia, H. Li and H. Huang, Korean J. Chem. Eng., 2011, 28, 99–105 CrossRef CAS.
- A. Yamaguchi, N. Hiyoshi, O. Sato and M. Shirai, Green Chem., 2011, 13, 873–881 RSC.
- M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS.
- L. Dosen-Micovic and Z. Cekovic, J. Phys. Org. Chem., 1998, 11, 887–894 CrossRef CAS.
- M. Kurszewska, E. Skorupowa, J. Madaj, A. Konitz, W. Wojnowski and A. Wisniewski, Carbohydr. Res., 2002, 337, 1261–1268 CrossRef CAS.
- A. Duclos, C. Fayet and J. Gelas, Synthesis, 1994, 1087–1090 CrossRef CAS.
- M. Gu, D. Yu, H. Zhang, P. Sun and H. Huang, Catal. Lett., 2009, 133, 214–220 CrossRef CAS.
- J.-J. Xia, D.-H. Yu, Y. Hu, B. Zou, P. Sun, H. Li and H. Huang, Catal. Commun., 2011, 12, 544–547 CrossRef CAS.
- Z. Cekovic, J. Serb. Chem. Soc., 1986, 51, 205–211 CAS.
- J. Li, PhD thesis: Lignocellulosic biomass conversion into platform chemicals in molten salt hydrate media, TU Delft, 2012, ch. 6 (in English), ISBN 978-90-9027073-9 Search PubMed; J. Li, W. Buijs, J. A. Moulijn and M. Makkee, submitted.
- L. W. Wright and J. D. Brandner, US Patent, 3023223, 1962 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
