 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emerging catalytic processes for the production of adipic acid
Stijn
Van de Vyver
and
Yuriy
Román-Leshkov
*
Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Room 66-456, Cambridge, Massachusetts 02139, USA. E-mail: yroman@mit.edu; Fax: +1 617-258-5042
First published on 30th January 2013
Abstract
Research efforts to find more sustainable pathways for the synthesis of adipic acid have led to the introduction of new catalytic processes for producing this commodity chemical from alternative resources. With a focus on the performance of oxygen and hydrogen peroxide as preferred oxidants, this minireview summarizes recent advances made in the selective oxidation of cyclohexene, cyclohexane, cyclohexanone and n-hexane to adipic acid. Special attention is paid to the exploration of catalytic pathways involving lignocellulosic biomass-derived chemicals such as 5-hydroxymethylfurfural, D-glucose, γ-valerolactone and compounds representative of lignin and lignin-derived bio-oils.
1. Introduction
Adipic acid (AA), also referred to as hexanedioic acid, is one of the most produced commodity chemicals worldwide. With a projected global market size of more than 6 billion pounds by 2017,1 AA is known to be a versatile building block for an array of processes in the chemical, pharmaceutical and food industries.2 Its primary use is as a precursor for the synthesis of the polyamide Nylon-6,6. Additionally, AA is widely used for the production of polyester and polyurethane resins, as a plasticizer in the production of polyvinyl chloride (PVC) and polyvinyl butyral (PVB), and as an approved additive in cosmetics, gelatins, lubricants, fertilizers, adhesives, insecticides, paper and waxes. Notably, approximately 75 years after DuPont's development of the first commercial AA process,2 long-standing interest in the improvement of its synthesis strategy continues to inspire the catalytic community to explore new processes and resources.The current industrial process for the production of AA relies on the catalytic oxidation of a mixture of cyclohexanol and cyclohexanone, also referred to as KA oil (6 × 106 tons per year).3 A recent review by Cavani and Alini describes different methods to produce the KA oil, the most common being the cobalt-catalyzed oxidation of benzene-derived cyclohexane with air (Scheme 1).4 Unfortunately, owing to the inverse dependence of the selectivity on cyclohexane conversion, this bulk oxidation is run at very low conversions (4–8%), thereby requiring distillation and recycling of unconverted cyclohexane.5–7 The second step of the process involves the oxidation of the KA oil with an excess of HNO3 in the presence of copper(II) and ammonium metavanadate catalysts. Studies by Aellig et al. have demonstrated that cyclohexanol likely reacts with HNO2 to form reactive (H)NOx species, suggesting that cyclohexanol is an essential co-substrate as pure cyclohexanone would be rather inert under the same conditions.8–10 A serious drawback of the nitric acid oxidation reaction is the stoichiometric reduction of HNO3 to NOx and the greenhouse gas nitrous oxide (N2O), the latter accounting for approximately 5–8% of the worldwide anthropogenic N2O emissions.11 Various technologies have been implemented by major AA producers to recover and reuse N2O or to thermally decompose it into O2 and N2.12 For example, BASF has patented a process for preparing cyclododecanone by the oxidation of cyclododecene with N2O.13,14 Another option is to integrate the use of the N2O-containing stream for the hydroxylation of benzene to phenol,15–17 which can then be hydrogenated into cyclohexanol to complete the N2O cycle.4 In addition to the downstream approaches compatible with the industrial practice, there has been a recent surge of interest in the development of alternative catalytic processes to expand the feedstock source for AA production.
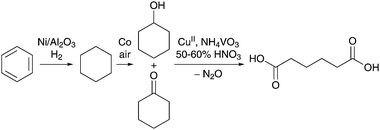 | ||
| Scheme 1 Simplified reaction scheme of the current industrial process for AA production by catalytic oxidation of KA oil with nitric acid. | ||
This review focuses on the advances in this rapidly emerging field, including mechanistic insights into oxidation processes and their implications on catalyst and process design. Rather than providing a comprehensive survey, we discuss examples that highlight opportunities and challenges in the synthesis of AA from the new generation of alternative substrates: cyclohexene, cyclohexane, cyclohexanone, n-hexane and lignocellulosic biomass-derived chemicals. Although the synthesis of AA and its precursors has also been demonstrated by the biocatalytic conversion of vegetable oils18,19 and α-ketoglutaric acid,20 by bis-hydroformylation or two-step carbonylation of 1,3-butadiene,21–23 and by dicarbonylation of 1,4-dimethoxy-2-butene,24 a discussion of these processes falls outside the scope of this minireview.
2. Oxidation of cyclohexene
The use of cyclohexene as a viable substrate for AA production has attracted much attention during the last 15 years, largely on account of increased cyclohexene availability by hydrogenation of benzene,25–27 or by dehydrogenation of cyclohexane.28–30 Two oxidants, i.e. hydrogen peroxide (H2O2) and molecular oxygen, are currently gaining momentum to replace nitric acid in the oxidation processes and thus to avoid N2O production. Research in this area has been extensively reviewed;4,12,31,32 therefore, only selected achievements will be presented here.2.1 Hydrogen peroxide as an oxidant
The first successful oxidation of cyclohexene to AA was reported in a seminal contribution by Sato et al.,33 who developed a biphasic system using 30% H2O2 in the presence of Na2WO4 as a homogeneous catalyst and [CH3(n-C8H17)3N]HSO4 as a phase-transfer catalyst (Scheme 2). Because cyclohexene and water are almost immiscible, their mixture creates a two-phase system containing the tungstate and H2O2 in the aqueous phase, and the phase-transfer catalyst in the organic cyclohexene phase. At a temperature of 75 to 90 °C, Na2WO4 is oxidized by H2O2 into an anionic peroxo species that is extracted by the quaternary ammonium cation into the organic phase.34 The reaction with cyclohexene restores the reduced form of the catalyst and returns it into the aqueous phase to initiate a new catalytic cycle.4 The solvent- and halide-free process affords analytically pure AA crystals in yields of 90% after 8 h of reaction. Various studies indicate that the reaction pathway for this transformation consists of six steps, involving three kinds of oxidation reactions (olefin epoxidation, alcohol oxidation and Baeyer–Villiger oxidation) and two hydrolytic reactions (vide infra).33,34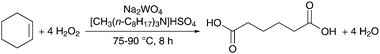 | ||
| Scheme 2 Oxidation of cyclohexene to AA with H2O2.33 | ||
The industrial applicability of the phase-transfer catalyst was later called into question by Deng et al. who noted that, besides being relatively expensive systems, the quaternary ammoniums also have a negative environmental impact.35 This inspired the development of a less harmful approach in which the ammonium salts were substituted by less expensive organic acid ligands such as oxalic acid, notably without affecting the yield and selectivity of the cyclohexene to AA conversion. A point of contention is the real configuration of the active mononuclear tungsten peroxo complex in the catalytic cycle (Fig. 1). A combined experimental and density functional theory (DFT) study indicates that the energy barriers of the catalytic cycle can be markedly reduced for the structures with oxalic acid as a ligand, suggesting that structures (ii) and (iv) should be easier to recycle.36 Moreover, theoretical results supported by X-ray crystal data favor a mechanism in which the peroxo ring structure is the active species for the oxidation reaction, instead of the originally hypothesized hydroperoxo structure.37
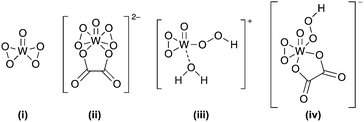 | ||
| Fig. 1 Proposed structures of tungsten peroxo and hydroperoxo complexes with and without an oxalic acid ligand.36 | ||
Faced with the problem of reactant incompatibility, Bohström et al. demonstrated the conversion of cyclohexene to AA in aqueous dispersions of mesoporous oxides with built-in catalytic WO3 sites.38 Alternatively, microemulsions made of benzalkonium chloride surfactants have been used to avoid the need for phase-transfer agents by providing a homogeneous reaction medium for cyclohexene as the oil phase and H2O2 as the aqueous phase.39,40 Several other tungstate-based catalysts have been reported as well, including oxotungsten-containing SBA-15,41 surfactant-type peroxotungstates,42 [BMIm]2WO4 supported on silica sulphamic acid,43 [WO(O2)2·2QOH],44 H3PW12O40,45 and combinations of Na2WO4 with H2SO446 or H2WO4 with acidic resins,47 thus emphasizing the remarkable catalytic efficiency of tungstate in this oxidation reaction.
As part of the ongoing effort to understand the mechanistic pathway of the oxidative cleavage reaction, Lee et al. explored titanium framework-substituted aluminophosphate (TAPO-5) as another catalyst for the oxidation of cyclohexene to AA.48 Nuclear magnetic resonance (NMR) and gas chromatography coupled with mass spectrometry (GC-MS) analyses provided insight into the formation of intermediates and shed light on the mechanism of the overall reaction (Scheme 3). In contrast to what was observed by Sato et al.,33 the analytical measurements revealed the formation of trans- and cis-stereoisomers of 1,2-cyclohexanediol during the progress of the reaction. These results suggest that while the trans-diol is known to be formed by the acid-catalyzed ring opening of the epoxide, the production of the cis-diol must proceed through a free-radical mechanism. It was also found that the TAPO-5 catalyst reacted significantly faster with the cis-diol than with its trans-analogue.
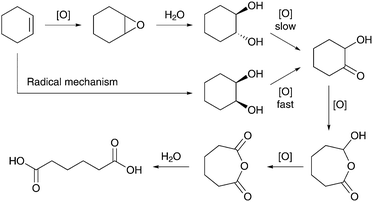 | ||
| Scheme 3 Mechanistic pathways for the oxidation of cyclohexene to AA in the presence of a TAPO-5 catalyst. Adapted from ref. 48. | ||
The insights gained into the mechanistic pathway have set forth the rational design of bifunctional heterogeneous catalysts containing Brønsted acid and metal sites as the primary way of further improving AA yields. For example, Ti-based catalysts have been developed by incorporating Al into the framework of mesostructured SBA-15, followed by Ti grafting.49,50 AA was synthesized in yields higher than 80% with tert-butyl hydroperoxide (t-BHP) as oxidant under mild, stoichiometric conditions (80 °C). Lapisardi et al. demonstrated the importance of acidity of the SBA-15-support for the catalytic activity of these materials.49 In this regard, a high Brønsted acid strength has also been shown to increase the rate of acid-catalyzed epoxide opening during the oxidation of cyclohexene to AA over zeolite occluded manganese diimine complexes.51
Other recent work has targeted the oxidant efficiency and the leaching of Ti species from the catalyst. Considerable effort has been devoted to improve the epoxidation step of cyclohexene by using Ti-grafted mesoporous silica catalysts.52,53 In order to reduce the unwanted decomposition of the oxidant, dropwise addition of H2O2 has been implemented to minimize its local concentration near the Ti sites.53 Encouraging results show that this method not only suppresses the disproportionation of H2O2 into O2 and H2O, but also favors the more selective heterolytic pathway of cyclohexene epoxidation instead of the less selective homolytic pathway (Scheme 4).54 Although not yet proven, lower water concentrations could reduce the hydrolytic cleavage of Ti–O–Si bonds of the grafted moieties and thus the aggregation of Ti isolated sites into larger and less active TiO2-like clusters. Timofeeva et al. attributed the leaching of Ti from a mesoporous titanium–silicate catalyst (Ti-MMM-2)55 to the interaction of surface Ti sites with the AA reaction product.56
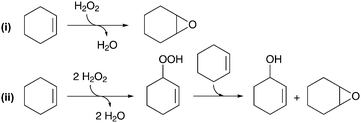 | ||
| Scheme 4 Mechanistic pathways for (i) heterolytic and (ii) homolytic epoxidation of cyclohexene with H2O2.54 | ||
2.2 Developments in process and reactor design
Laboratory-scale experiments for producing AA by H2O2-mediated oxidation of cyclohexene have traditionally been carried out in the batch mode using either glass vessels or round-bottom flasks. However, along with the development of more efficient catalysts, breakthroughs have been achieved in the reaction engineering of this process. Freitag et al. reassessed the biphasic catalytic system based on Na2WO4 and [CH3(n-C8H17)3N]HSO4 in order to evaluate the use of microwave irradiation as an alternative heating source, and the substitution of Na2WO4 by commercially available tungsten and molybdenum salts.57 Using Na2WO4, the highest AA yield (68%) was achieved after 90 minutes of reaction under microwave conditions. Besides the drastic reduction in reaction time, they reported competitive yields when using sodium polytungstate as the oxidation catalyst.Alternatively, Buonomenna et al. investigated the application of polymeric microporous membrane reactors to avoid the need for phase-transfer catalysts.58 Compartmentalization of the organic phase containing cyclohexene and the aqueous phase containing the oxidant, the catalyst and succinic acid enabled the production of AA in yields up to 90%. Importantly, the use of succinic acid improved the contact between the catalytic active sites and the cyclohexene reagent. Preliminary results obtained with an ammonium molybdate catalyst ((NH4)6Mo7O24) are encouraging to exploit such modular design to scale-up the process by interfacing several membrane contactors in series or in parallel. However, the traditional high cost of membrane reactors could be problematic for larger-scale production.
A successful example of a large-scale process for the H2O2-mediated oxidation of cyclohexene has most recently been published by Wen et al.59 The implementation of continuous-flow techniques as an alternative to traditional batch oxidations has made it possible to optimize the reaction parameters for a catalyst combination consisting of H2WO4, H2SO4 and H3PO4. An in situ synthesized species, i.e. {PO4[WO(O2)2]4}3−, was suggested to be the true active species, whereas experimental results showed that it can be reversibly converted into soluble H3PW12O40 by the consumption of H2O2. The feasibility of the continuous-flow process was demonstrated in a reaction device consisting of four 5000 L continuous stirred-tank reactors (CSTR) connected in series, representing a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold scale-up from the laboratory-scale reaction. The scale-up to the pilot plant showed excellent results for both the product yield (94.1% at lab-scale vs. 94.7% at pilot-scale) and the purity of the crude AA product (98.8% at lab-scale vs. 99.0% at pilot-scale). To the best of our knowledge, this is the first example of a H2O2-based process for the production of AA from cyclohexene that is approaching a stage at which it becomes a realistic alternative for the current industrial process.
000-fold scale-up from the laboratory-scale reaction. The scale-up to the pilot plant showed excellent results for both the product yield (94.1% at lab-scale vs. 94.7% at pilot-scale) and the purity of the crude AA product (98.8% at lab-scale vs. 99.0% at pilot-scale). To the best of our knowledge, this is the first example of a H2O2-based process for the production of AA from cyclohexene that is approaching a stage at which it becomes a realistic alternative for the current industrial process.
Conceptually, Vural Gürsel et al. have initiated a rethinking of the process design based on the use of microreactor technologies and flow chemistry as intensification fields.5–7,60 Simulations were performed to compare the flow schemes of the commercial route (Scheme 1) with the synthesis route proposed by Sato et al. (Scheme 2), the latter being modelled as a continuous process with microreactors. The results of this technoeconomic evaluation demonstrate that the one-step process leads to reduced capital cost requirements owing to a more compact plant design (i.e., only one reactor and a reduced number of downstream units). A comparison of the utility requirements shows that although the microreactor costs are higher than the batch reactor costs, the drastic simplification of the process results in about 30% less energy consumption, in particular by avoiding the need for energy-intensive separation units. Currently, expansion of this methodology is in progress to estimate the environmental impact of the process by means of a Life Cycle Assessment (LCA). The expertise built up in this line of research has also led to a new study on the implementation of flow units into modular plant environments such as so-called container plants.6
3. Oxidation of cyclohexane
3.1 Air or oxygen as an oxidant
Sporadic reports over the past decades have proposed the direct oxidation of cyclohexane to AA using Co and Mn catalysts with acetic acid as the solvent.61–65 Inspired by this work, Bonnet et al. investigated the combination of lipophilic carboxylic acids and low loadings of Co and Mn salts.66 The choice for lipophilic carboxylic acids was motivated by the need to facilitate their recycling after partitioning of AA in the aqueous phase (Fig. 2). A screening of several combinations led to the selection of 4-tert-butylbenzoic acid with ppm levels of Co and Mn as the most effective system. The applicability of the method was further demonstrated in semi-batch and continuous-flow experiments in a 1 L reactor under 20 bar air, resulting in a maximum selectivity of 71% AA and a productivity of 95 g L−1 h−1.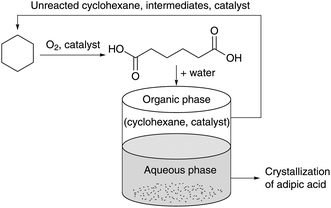 | ||
| Fig. 2 Schematic diagram of the process for oxidation of cylcohexane to AA and recycling of the lipophilic catalyst. Adapted from ref. 66. | ||
Nanostructured gold-based catalysts have also been the subject of numerous studies on the aerobic oxidation of cyclohexane. Examples include Au/graphite,67 Au/Al2O3,68,69 Au nanoparticles on Ti-doped SiO2,70 Au/TiO2,68 Au/MCM-41,71 Au/SBA-15,68,72 and Au/C.73 The main oxidation products obtained with these catalysts were cyclohexanol and cyclohexanone, although AA has occasionally been observed as a byproduct at high conversions.68 Interestingly, however, Alshammari et al. recently showed that the gold-catalyzed oxidation of cyclohexane to AA is possible to some extent by fine-tuning the particle size and dispersion of Au nanoparticles on TiO2.74 Using acetonitrile as solvent and t-BHP as an initiator, the highest AA yield was 8% after 4 h of reaction at 150 °C and 10 bar of O2.75
In 2011, Yu et al. approached the field from a new angle by pioneering the use of multiwalled carbon nanotubes (CNTs) as a metal-free catalyst for the aerobic oxidation of cyclohexane.76 The performance of nitrogen-doped CNTs was demonstrated to even exceed the activity of most Au-based catalysts. For instance, after reacting for over 8 h at 125 °C and 15 bar of O2, N-doped CNTs gave 45% conversion with 60% AA selectivity. The inhibition of catalytic activity in the presence of p-benzoquinone as a radical scavenger indicated that this CNT-catalyzed oxidation reaction proceeds through a similar radical-chain mechanism as proposed for the liquid-phase autoxidation of cyclohexane. Beyond the well-known radical-chain sequence (reactions (1) to (5)), Hermans et al. provided solid evidence that a concerted bimolecular reaction of cyclohexyl hydroperoxide with cyclohexanone (reaction (6)) causes a predominant initiation process in the autoxidation of cylcohexane.3,77,78
| C6H11OOH → C6H11O˙ + HO˙ | (1) |
| C6H11O˙ + C6H12 → C6H11OH + C6H11˙ | (2) |
| C6H11˙ + O2 → C6H11OO˙ | (3) |
| C6H11OO˙ + C6H12 → C6H11OOH + C6H11˙ | (4) |
2C6H11OO˙ → C6H11OH + C6H10(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) + O2 O) + O2 | (5) |
C6H11OOH + C6H10(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) → C6H11O˙ + C6H9( O) → C6H11O˙ + C6H9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)−αH + H2O O)−αH + H2O | (6) |
Indeed, the apparent activation energy for the CNT-catalyzed cyclohexane to AA conversion was found to be (111.5 ± 15.5) kJ mol−1, which is consistent with the value for reaction (6) calculated by transition-state theory (116.3 kJ mol−1). Yu et al. demonstrated that such unique reactivity is imparted by the ability of CNTs to accept and stabilize cyclohexyl hydroperoxide radicals, coupled with a facilitated adsorption of reactive intermediates due to the presence of electron-donating nitrogen species at the exposed graphitic sites. Besides the observed catalytic effects, the stabilization of peroxyl radicals by π–π conjugation with the CNTs was corroborated by a theoretical calculation performed at the B3LYP level of DFT.
Follow-up studies have achieved the modification of the electronic characteristics of the CNTs by filling their interior with Fe nanowires.79 This can be accomplished by growing the CNTs through chemical vapor deposition with ferrocene as the catalyst and a mixture of xylene and dichlorobenzene as the carbon source. The resulting encapsulated Fe not only allows an easy magnetic separation of the catalyst, but it also helps to enhance the oxidative activity of the CNTs by increasing the electron density of its external surface. The best results were obtained for a high Fe content of 19%, achieving a maximum conversion of 37% with 61% AA selectivity after 8 h of reaction. Although this concept is still in its infancy, it provides an elegant approach for carbon-catalyzed oxidations with obvious advantages such as low cost and facile catalyst recovery.
3.2 Examples of biomimetic approaches
A valuable example of supramolecular chemistry in the context of biomimicking is the catalytic oxidation of cyclohexane by iron-phthalocyanine (FePc, Fig. 3i) encapsulated in crystals of zeolite Y.80,81 FePc is of particular interest as a mimic of the active site of enzyme cytochrome P-450. The planar FePc complex can be encapsulated in the supercages of zeolite Y by the so-called “ship-in-a-bottle” synthesis method. The role of the zeolite is similar to that of the proteic mantle of an enzyme, i.e., imposing constraints on the active site and protecting it against oxidative destruction. For the oxidation of cyclohexane, Thibault-Starzyk et al. found that the use of a polar solvent was crucial to dissolve AA and to extract it from the zeolite pores.81 For instance, a yield of 30% AA at 85% conversion of cyclohexane was obtained by heating a mixture of the catalyst with acetone, t-butanol, cyclohexane and t-BHP at 60 °C. By adding acetone as a co-solvent, the authors sought to find a compromise in polarity to improve the diffusion of cyclohexane in the relatively hydrophilic zeolite without inhibiting the approach of the apolar substrate. Related work by Parton et al. extended this concept by embedding the catalyst in a polydimethylsiloxane (PDMS) membrane.82 The hydrophobicity of this polymer accounted for a four-fold increase in the catalytic activity, likely attributable to an enhanced concentration of cyclohexane as well as a decreased concentration of t-BHP in the PDMS matrix. Whether the zeolite-encaged iron complex is also stable and reusable under AA synthesis conditions remains to be examined.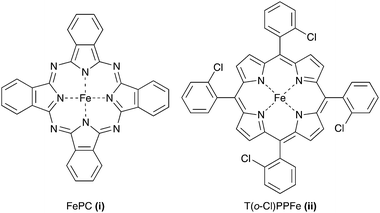 | ||
| Fig. 3 Atomic structure of (i) iron-phthalocyanine and (ii) meso-tetra (o-chlorophenyl) iron porphyrin. | ||
A second biomimetic attempt was published by Yuan et al., who disclosed the solvent-free oxidation of cyclohexane using meso-tetra (o-chlorophenyl) iron porphyrin (T(o-Cl)PPFe, Fig. 3ii) as a homogeneous catalyst.83 Interesting features of this catalyst are the high turnover numbers and the relatively mild reaction conditions (140 °C, 25 bar of O2). However, as far as the production of AA is concerned, the highest yield was only 21%. Noack et al. noted that, under these conditions, the performance of the iron porphyrin catalyst is likely limited by overoxidation of AA, leading to the formation of glutaric and succinic acid.84 Hybrid DFT calculations point towards a mechanism involving an FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species formed by the reaction of FeII with O2 (Scheme 5). The first step of the mechanism entails the catalytic transformation of cyclohexane to 1,2-cyclohexanediol via two consecutive hydroxylation reactions, each initiated by a hydrogen-atom transfer from the substrate to the FeIV
O species formed by the reaction of FeII with O2 (Scheme 5). The first step of the mechanism entails the catalytic transformation of cyclohexane to 1,2-cyclohexanediol via two consecutive hydroxylation reactions, each initiated by a hydrogen-atom transfer from the substrate to the FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxidant. Subsequently, 1,2-cyclohexanediol undergoes an intradiol C–C bond cleavage into adipaldehyde and an oxidation to AA.
O oxidant. Subsequently, 1,2-cyclohexanediol undergoes an intradiol C–C bond cleavage into adipaldehyde and an oxidation to AA.
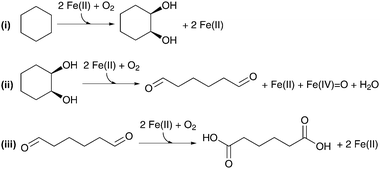 | ||
| Scheme 5 Major steps involved in the heme-catalyzed oxidation of cyclohexane to AA. Adapted from ref. 84. | ||
4. Oxidation of cyclohexanone
In 1958, a contribution by Starcher and Phillips had already demonstrated the formation of small amounts of AA (1%) as a side reaction in the Baeyer–Villiger oxidation of cyclohexanone with peracetic acid.85 However, it was almost half a century later that the direct oxidation of cyclohexanone aroused the interest of researchers looking for new synthesis routes for AA. In the past, various oxidants have been used for the oxidation of cyclic ketones, including HNO3, KMnO4, CrO3, KO2, O2, and H2O2. The first four candidates in this series are clearly less interesting from a “green” chemistry perspective,86 and will therefore not be the focus of this minireview.4.1 Air or oxygen as an oxidant
Besson et al. reported the use of metal-free activated carbon for the oxidation of cyclohexanone using air instead of pure oxygen.87 The catalysts were prepared by polycondensation of Novolac phenolic resins with cross-linking agents and ethylene glycol as solvent. The full conversion of cyclohexanone at 140 °C and 50 bar of air led to moderate yields of C4–C6 dicarboxylic acids: AA (15–34%), glutaric acid (23–32%) and succinic acid (15–23%). The oxidation activity mainly depended on the surface area and the microporosity of the carbon catalysts, both of which could be increased by activation of the catalysts under CO2 or air. Shortly afterwards, the authors examined the role of surface oxygen functionalities on the catalytic behavior of the carbon materials.88 Notably, a heat treatment under N2 at 900 °C selectively eliminated the quinone groups, leading to a decreased AA selectivity. Hence, the quinone groups were postulated to be the active sites for the activation of molecular O2. In further investigations, the incorporation of phosphorus into the carbon matrix showed a slight selectivity increase to AA.89 The results indicated that a high density of oxygenated functional groups on the modified catalyst had a promoting effect on the oxidative bond breaking of the cyclic ketone. Similarly, the use of carbon coated monolithic catalysts for the oxidation of cyclohexanone to AA by air has also shown promising results.90 Crezee et al. succeeded in extending the scope of the reaction to slurry phase operation. The deposition of highly dispersed Pt enhanced the selectivity to AA at 140 °C from 9 to 21%. Further investigations are required to fully understand and exploit the role of the carbon and platinum sites to maximize product selectivity.While others considered individual Co and Mn acetates,91 Chavan et al. introduced μ3-oxo-bridged Co and Mn cluster complexes for the oxidation of either pure cyclohexanone or mixtures of cyclohexanone and cyclohexanol with air.92 For example, cyclohexanone was oxidized to AA with CoMn2(μ3-O)(OAc)6(py)3 at 100 °C and 38 bar of air in yields of nearly 85%. Unfortunately, significant leaching of the metal ions was observed for catalytic reactions with the zeolite-Y-encapsulated form of the cluster complexes. In situ electronic and electron paramagnetic resonance (EPR) spectroscopic studies revealed a partial decomposition of the cluster complexes to monomeric CoII and MnII species during the oxidation reaction.
Regarding the solvent, there is considerable evidence that the use of acetic acid can affect the prevailing mechanism by which cyclohexanone is transformed into AA. For example, Cavani et al. investigated the reactivity of homogeneous Keggin-type P/Mo/V polyoxometalates (POM) for the oxidation of cyclohexanone with O2.93 Using a water-only solvent, or in the presence of acetic acid but with a high catalyst to substrate ratio, it was shown that the oxidation proceeds mainly through a redox-type mechanism with direct involvement of the POM. On the other hand, for reactions carried out in the presence of acetic acid and at comparatively low catalyst to substrate ratios, the prevailing mechanism is suggested to be a radical-chain autoxidation. Variations in the solvent composition demonstrated not only an increase of the reaction rate, but also revealed an important effect on the product distribution. While the redox mechanism appears to be more selective to AA, the radical-chain autoxidation leads to parallel oxidative degradation reactions with formation of significant amounts of glutaric and succinic acid.
Until recently, tin containing microporous materials were mainly known as efficient catalysts for the Baeyer–Villiger oxidation of ketones by H2O2.94,95 However, research by Dutta et al. has shown the advantages of a new porous organic–inorganic hybrid tin phosphonate (HMSnP-1) material as a heterogeneous catalyst for the aerobic oxidation of cyclohexanone to AA.96 Their study demonstrates AA yields up to 74% for oxidations in aqueous media. In contrast to a previously reported mesoporous tin phosphate,97 the presence of free amines in the spacer group of as-synthesized HMSnP-1 seems particularly useful for stabilization of the keto–enol tautomer. The oxidation reaction is further enhanced upon the activation of molecular O2 by Sn incorporated in the porous framework (Scheme 6). Subsequent hydrolysis of ε-caprolactone leads to the formation of 6-hydroxohexanoic acid, which is finally oxidized to AA. Interestingly, the use of methanol as solvent afforded hydroxomethyl hexanoate as the main reaction product. The incomplete oxidation of cyclohexanone is likely due to the lower solubility of molecular O2 in methanol.
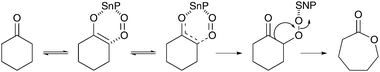 | ||
| Scheme 6 Proposed mechanism for the transformation of cyclohexanone to ε-caprolactone over HMSnP-1 in the presence of O2.96 | ||
4.2 Hydrogen peroxide as an oxidant
In an extension of their work on cyclohexene oxidation, Usui and Sato accomplished an organic solvent- and halide-free oxidation of cyclohexanone with aqueous 30% H2O2 and H2WO4 as a catalyst.98 An advantage of this method is the ease of operation, even at a hectogram-scale. For example, an almost quantitative yield of AA could be achieved for the reaction of 100 g cyclohexanone with 382 g H2O2 (30%) and 2.50 g H2WO4 after 20 h at 90 °C. The high AA yields are attributed to the pKa value of 0.1 and the good solubility in water of the catalytic active species, H2[WO(O2)2(OH)2], that is formed from oxidation of H2WO4 with H2O2. Remarkably, none of the proposed oxidation intermediates could be detected by GC or 1H NMR analysis in the course of the reaction. The authors ascribe this observation to the fact that the Baeyer–Villiger oxidation of cyclohexanone is probably the rate-determining step in the conversion to AA. The Baeyer–Villiger reaction itself proceeds in two substeps: (i) the addition of H2O2 to the carbonyl group of cyclohexanone to form a tetrahedral intermediate, known as the Criegee intermediate, and (ii) a concerted rearrangement of the tetrahedral adduct to give ε-caprolactone with water as the only byproduct (Scheme 7).99,100 However, it is not yet clear whether the first or the second substep in the Baeyer–Villiger mechanism is rate-limiting and if the acid catalyst increases the rate of either substep.99 Under comparable conditions, the oxidation of five- to eight-membered cycloalkanones and cycloalkanols gave the corresponding dicarboxylic acids with yields ranging from 81% (heptanedioic acid) to 98% (glutaric acid).98 Note that linear ketones could not be converted to the corresponding carboxylic acids.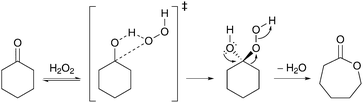 | ||
| Scheme 7 Baeyer–Villiger oxidation of cyclohexanone with H2O2. | ||
Recently, while studying the Baeyer–Villiger oxidation of cyclohexanone with H2O2 to ε-caprolactone, Cavani et al. found that a hitherto overlooked radical-mediated reaction can lead to the concurrent formation of AA.101 Their work initially focused on the thermal, uncatalyzed oxidation of cyclohexanone with H2O2 at 90 °C, which surprisingly afforded yields of 22% AA after 6 h of reaction time. Early mechanistic studies suggested three different pathways for the activation of cyclohexanone: (i) by addition of H2O2 (Scheme 7), (ii) by hydrogen bonding of the carbonyl group with water, or (iii) by a nonconventional radical reaction with hydroxyl radicals generated by decomposition of H2O2 (reactions (7) and (8)).
| H2O2 → 2HO˙ | (7) |
| HO˙ + H2O2 → H2O + HO2˙ | (8) |
It was argued that the subsequent conversion of ε-caprolactone to AA or C4–C5 diacids most likely proceeds by a reaction with the radical species formed in reactions (7) and (8). In fact, when using titanium silicalite-1 (TS-1) under similar conditions, the authors observed a significant increase in the consumption of H2O2. Further investigations led to the hypothesis that Ti-OOH species could act as a reservoir for hydroxyl radicals through controlled decomposition of the hydroperoxo species.
4.3 Synthesis of dibutyl acidic acid esters
One recent study considered the possibility of synthesizing dicarboxylic esters by performing the H2O2-mediated oxidation of cyclohexanone in an alcoholic solution.102 The catalytic process reported by Terent'ev et al. is based on their previous reports demonstrating (i) the synthesis of geminal bishydroperoxides from cyclic ketones and H2O2 in tetrahydrofuran (THF) at 15–20 °C,103 and (ii) the oxidation of bishydroperoxides at higher temperatures to form dicarboxylic esters.104 It was found that the following conditions are essential to produce the dibutyl esters by one-pot oxidation of cyclohexanone in butanol: a temperature higher than 80 °C, a concentration of H2SO4 of 0.2–1 mol L−1 and a molar ratio of cyclohexanone to H2O2 of 5–10.5. Oxidation of n-hexane
From a catalyst design perspective, perhaps one of the most impressive accomplishments to date is the oxidation of n-hexane to AA by using molecular sieve catalysts.105 The selective oxyfunctionalization of the terminal methyl groups in alkanes has already been demonstrated by enzymes with non-heme iron active centers, yet attempts to use synthetic materials to mimic their properties have generally been met with only limited success.4 To this end, controlled oxidation with molecular sieves offers a promising alternative route with significant advantages from a processing standpoint.The vast body of research developed by Thomas and Raja et al. shows that Co(III)- and Mn(III)aluminophosphates (AlPOs) act as regioselective catalysts for the oxidation of alkanes by molecular O2.105–108 The key characteristics of these molecular sieves are the well-defined sizes and shapes of the eight-ring windows that allow only end-on entries of the alkanes into the cavities containing isolated CoIII and MnIII ions at the inner walls. In the case of n-hexane oxidation, the positioning of the tetrahedrally coordinated CoIII ions in the AlPO is another important consideration, as they function as centers for the generation of free radicals,108,109 and play an important role in directing the preferential attack by O2 on the terminal group of the alkane. For the oxidation of n-hexane, Raja et al. asked whether it would be possible to accommodate two CoIII ions in each cage of an AlPO framework, preferably opposite to each other, in order to achieve selective oxyfunctionalization at both ends of the alkane.105 Although the precise distribution of the CoIII ions could not be determined by direct electron microscopy, computational estimates hinted at the tendency of two CoIII ions to be situated in each cage for Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios of 0.08 or higher. Among the different AlPO topologies examined, AlPO-18 and AlPO-34 proved the most effective for AA production. For example, CoAlPO-18 with a Co
P ratios of 0.08 or higher. Among the different AlPO topologies examined, AlPO-18 and AlPO-34 proved the most effective for AA production. For example, CoAlPO-18 with a Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 0.10 catalyzed the oxidation of n-hexane at 100 °C and 15 bar of air to AA with 33.6% selectivity and 9.5% conversion. With regard to the product distribution, the main byproducts were hexanoic acid and 2-hexanone, with selectivities up to 35.2% and 19.7%, respectively. After filtering the catalyst from the hot reaction mixture, less than 3 ppb of Co were found in the solution and no significant changes in either the n-hexane conversion or product selectivities could be observed, supporting the stability of the Co cations in the AlPO framework. However, Hartmann and Ernst later argued that leaching of active species by carboxylic acids was probably minimized by working at low conversions (<10%).110
P ratio of 0.10 catalyzed the oxidation of n-hexane at 100 °C and 15 bar of air to AA with 33.6% selectivity and 9.5% conversion. With regard to the product distribution, the main byproducts were hexanoic acid and 2-hexanone, with selectivities up to 35.2% and 19.7%, respectively. After filtering the catalyst from the hot reaction mixture, less than 3 ppb of Co were found in the solution and no significant changes in either the n-hexane conversion or product selectivities could be observed, supporting the stability of the Co cations in the AlPO framework. However, Hartmann and Ernst later argued that leaching of active species by carboxylic acids was probably minimized by working at low conversions (<10%).110
These and similar examples inspired the use of molecular sieves for other reactions such as the epoxidation of alkenes,111 the Baeyer–Villiger oxidation of ketones to lactones,112 or the selective oxidation of cyclohexane113–117 and benzene.118 Among the issues that remain to be addressed are the long-term stability of the molecular sieve catalysts,110 the structural and functional characterization of the metal species,119 and a more detailed description of the kinetics and mechanisms by which the metal-substituted AlPOs catalyze the oxidations.113 Gaining mechanistic insights into the elementary steps of alkane oxidation cycles is far from trivial, as many of the proposed intermediates cannot be directly detected. Gómez-Hortigüela et al. have now published a series of papers on computational analysis of the complete reaction mechanism for the aerobic oxidation of hydrocarbons catalyzed by Mn-doped AlPO-5,120 including the four subsequent stages of the catalytic cycle: preactivation of the Mn sites,121 hydroperoxide decomposition,122 propagation,123 and catalyst regeneration.124 This complementary information makes the complex free-radical pathways more straightforward to understand and helps to interpret basic experimental observations. It is interesting to note that Lü et al. most recently confirmed the radical mechanism for the aerobic oxidation of cyclohexane to AA using an Anderson-type POM catalyst.125
6. Catalytic pathways involving lignocellulosic biomass-derived chemicals
Instead of oxidizing one specific linear or cyclic C6 molecule, AA production could come from a more abundant and versatile feedstock such as lignocellulose. The conversion of biomass components, in particular cellulose, hemicellulose and lignin, is a current topic of intense scientific research.126–137 The industrial interest in biobased AA is reflected in the plans of firms such as DSM, Verdezyne, Rennovia, BioAmber, Amyris, Genomatica, Aemetis and Myriant to enter the AA market with a renewable alternative. In this section, we describe the current status and future directions in AA production from lignocellulosic biomass-derived chemicals involving bio- and chemocatalytic methods.6.1 Conversion of 5-hydroxymethylfurfural
The past few years have seen a flurry of research into the production of 5-hydroxymethylfurfural (5-HMF) using diverse solvents, catalysts and biphasic systems for the processing of mono- and polysaccharides such as D-glucose, D-fructose, D-mannose, sucrose, cellulose, inulin, starch and xylan.138–147 Not surprisingly, multiple pathways have been proposed for the conversion of 5-HMF into AA. For example, already in 1981, Faber (Hydrocarbon Research Inc., Lawrenceville, NJ, US) patented a multistep process for AA synthesis based on lignocellulosic biomass-derived 5-HMF.148 The four steps in this process include: (i) acid-catalyzed hydrolysis/dehydration of lignocellulose to 5-HMF in aqueous solutions of H2SO4, (ii) RANEY®-Ni catalyzed hydrogenation of 5-HMF to 2,5-di-hydroxymethyl-tetrahydrofuran (DHMTHF), (iii) hydrogenolysis of DHMTHF to 1,6-hexanediol with a copper chromite catalyst in a fixed-bed reactor, and (iv) biocatalytic oxidation of 1,6-hexanediol (1,6-HD) to AA by certain bacteria belonging to the Pseudomonadaceae family (e.g., Gluconobacter oxydans). Further contributions to the catalytic hydrogenation of 5-HMF into DHMTHF were recently made by Alamillo et al.149 and Buntara et al.150 The latter authors confirmed the excellent performance of RANEY®-Ni in this reaction by achieving essentially quantitative yields of DHMTHF (cis/trans = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) after 14 h of reaction at 100 °C and 90 bar of H2 (Scheme 8).
2) after 14 h of reaction at 100 °C and 90 bar of H2 (Scheme 8).
 | ||
| Scheme 8 Hydrogenation of 5-hydroxymethylfurfural to 2,5-di-hydroxymethyl-tetrahydrofuran with RANEY®-Ni catalysts.150 | ||
Buntara et al. presented distinct, yet highly complementary, pathways for the subsequent conversion of DHMTHF to 1,6-hexanediol.150,151 Particularly interesting about their work is the Rh-Re/SiO2 catalyzed ring-opening reaction of DHMTHF into 1,2,6-hexanetriol (1,2,6-HT) and the conversion of 1,2,6-HT into tetrahydro-2H-pyran-2-ylmethanol (2-THPM, Scheme 9). Somewhat unexpectedly, it was found that the addition of Brønsted acids to the hydrogenation reaction of 1,2,6-HT led to its ring-closure in very high yields of 99% 2-THPM. Such results motivated the authors to explore the direct hydrogenolytic ring-opening of DHMTHF into 1,6-hexanediol by using a combination of Rh-Re/SiO2 and acid catalysts such as Nafion SAC-13. This one-pot catalytic process, performed at 120 °C and 80 bar of H2, allowed the full conversion of DHMTHF with selectivities to 1,6-hexanediol of up to 86%. Other solid acids were also capable of converting DHMTHF, albeit at slightly lower selectivities.
A discussion on this pathway should be taken as part of the general issue of C–O bond hydrogenolysis of polyols and cyclic ethers. Recent reports by Chia et al. and Chen et al. demonstrate that ReOx-promoted Rh/C catalysts are highly selective for the hydrogenolysis of 2-THPM into 1,6-hexanediol.152,153 The hydrogenolysis mechanism of secondary C–O bonds in cyclic ethers can be understood in terms of the bifunctional nature of the ReOx-promoted Rh/C catalyst, which is thought to facilitate both the acid-catalyzed ring-opening and dehydration of 2-THPM coupled with the metal-catalyzed hydrogenation (Scheme 10).152 By combining a highly reducible metal with an oxophilic metal, this approach could, in principle, be transferred to the deoxygenation of other biomass-derived cyclic ethers and polyols. Continued research into this and related hydrogenolysis reactions is needed and can be anticipated ultimately to advance a breakthrough in the pathway to AA.
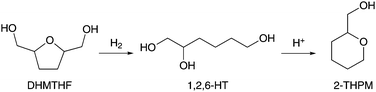 | ||
| Scheme 9 Consecutive reaction pathway for the conversion of 2,5-di-hydroxymethyl-tetrahydrofuran to tetrahydro-2H-pyran-2-ylmethanol via 1,2,6-hexanetriol.150 | ||
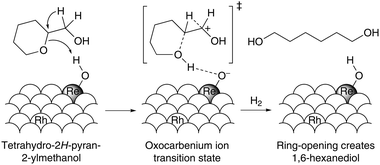 | ||
| Scheme 10 Schematic illustration of the active sites on rhodium-rhenium catalysts for the hydrogenolysis of 2-THPM. Adapted from ref. 152. | ||
In an alternative approach, Boussie et al. (Rennovia Inc. Menlo Park, CA, US) recently patented a two-step process for the production of AA from 5-HMF via 2,5-furandicarboxylic acid (FDCA).154 Previous work had focused on the synthesis of FDCA via catalytic oxidation of 5-HMF155–157 or dehydration/oxidation of fructose.158,159 As demonstrated for Au/CeO2 and Au/TiO2 catalysts, the reaction pathway for the aqueous-phase oxidation of 5-HMF is proposed to proceed via the formation of a hemiacetal, 5-hydroxymethyl-2-furancarboxylic acid and 5-formyl-2-furandicarboxylic acid as the key intermediates.160 The catalytic hydrogenation of FDCA with Pd/silica at 140 °C and 52 bar of H2 yielded 88% of tetrahydrofuran-2,5-dicarboxylic acid (THFDCA, Scheme 11).154 The inventors claim that Pd/silica and Rh/silica can hydrogenate THFDCA to AA in excellent yields as high as 99% after 3 h of reaction at 160 °C and 49 bar of H2. It is clear that the economic viability and environmental impact of this process have yet to be improved, as in both cases the catalysts require prohibitively high pressures, halogens and acetic acid as solvent. Regardless, it is tempting to speculate that furan derivatives might play a pivotal role in the future production of biobased AA.
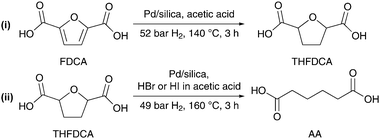 | ||
| Scheme 11 Two-step process for the catalytic hydrogenation and ring-opening of 2,5-furandicarboxylic acid to AA.154 | ||
6.2 Conversion of D-glucose
In parallel, Boussie et al. disclosed a second patent for the catalytic transformation of D-glucose to AA via glucaric acid.161 Commercialization of this pathway is being pursued by the US start-up firm Rennovia in Menlo Park, California. Preliminary cost estimates indicate that the catalytic process is economically advantageous over the petrochemical route when the price of crude oil is $50 per barrel.162 To make the innovation even more sustainable, the firm can benefit from using its oversupplied high fructose corn syrup (HFCS) as an initial feedstock source. Rennovia currently produces AA at a 1 lb h−1 pilot plant and plans to scale up to commercial quantities of 300 to 500 million lb per year by the end of 2013 or the beginning of 2014.Regarding the first step in this process (Scheme 12), catalyst screening showed that Pt catalysts were the most suitable for the oxidation of D-glucose.161 Even in the absence of any added base, Pt/silica afforded glucaric acid yields of 66% after 8 h of reaction at 90 °C and 5 bar of O2. Despite the good results, the examples provided herein refer only to reactions with 250 μL D-glucose solutions. Specifically, issues such as the pH effect and deactivation of the Pt catalysts during the oxidation of D-glucose remain of concern.163–166 The subsequent hydrodeoxygenation reaction could be achieved by using PdRh/SiO2 catalysts. In the presence of HBr and acetic acid, these bimetallic catalysts were capable of converting glucaric acid into AA in yields ranging from 50 to 77% after 3 h of reaction at 140 °C and 49 bar of H2. Unfortunately, again no mention is made of the catalyst stability and reuse. An additional scientific challenge lies in the elucidation of the role of the halogen source.161 It is assumed that during hydrodeoxygenation of glucaric acid, a secondary alcohol group reacts with HBr to form an alkyl bromide. The mechanism by which the C–Br bond converts to a C–H bond is not yet fully resolved but at least three plausible pathways have been proposed. First, the intermediate might react with H2 to form the C–H bond along with HBr. Second, the intermediate might undergo a dehydrobromination reaction to form an olefin that could be further reduced in the presence of PdRh/SiO2. In the third hypothesized pathway, a reaction of the brominated intermediate with HBr not only leads to the formation of the C–H bond, but also releases molecular bromine as a byproduct.
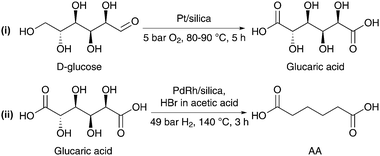 | ||
| Scheme 12 Two-step process for the conversion of D-glucose to AA via the formation of glucaric acid.161 | ||
In a biochemical alternative route, strains of Escherichia coli have been constructed to produce AA from D-glucose.167,168 The enzymatic method proceeds via an aerobic pathway in which D-glucose is transformed into the intermediates catechol and cis,cis-muconic acid, respectively. The conversion to AA had to be performed in a chemocatalytic step by hydrogenating cis,cis-muconic acid with Pt/C,167 bimetallic RuPt nanoparticles,169 or titania-supported Re catalysts.170 However, several enzymatic transformations are currently being developed that could pave the way towards a completely biological synthesis method.171–173 A consideration of the biotechnological production of AA, as well as the metabolic pathways leading to its precursors, has recently been reviewed by Polen et al.174
6.3 Conversion of γ-valerolactone
The ring-opening hydrocarboxylation of γ-valerolactone (GVL) represents another attractive pathway with considerable potential for the production of biobased AA. The precursor GVL can be produced by catalytic hydrogenation of levulinic acid,175–187 a platform chemical derivable from cellulose188–197 and/or hemicellulose fractions.198–200 As an illustrative example of the economic viability of GVL production, we draw attention to the technoeconomic analysis performed by Sen et al. for an integrated strategy to convert lignocellulose into GVL.201 A patent search reveals that many of the proposed strategies for AA synthesis require homogeneous rhodium or iridium catalysts in the presence of either a bromide or iodide promoter.202–205 For example, direct hydrocarboxylation of GVL has been performed with CO and small amounts of water in an acetic acid solution, using RhCl3 as a catalyst and HBr as a promoter.205 Product yields of 61% AA, 21% methylglutaric acid, 12% valeric acid and 4% ethylsuccinic acid could be achieved after 4 h of reaction under 28 bar of CO at 220 °C. Interestingly, under comparable conditions, the bromide-promoted rhodium catalyst was able to perform the isomerization of 2-methylglutaric acid to AA, as well as the interconversion of valeric acid to 2-methylbutyric acid.206 However, the daunting reaction conditions needed for the direct hydrocarboxylation of GVL have recently stimulated the development of new multistep process designs.Partly related to the work of Bond et al. on GVL ring-opening,207 Wong et al. (Institute of Chemical and Engineering Sciences, Singapore) advanced a three-step catalytic process for AA production, comprising: (i) reactive distillation of GVL in the presence of an acid catalyst such as SiO2–Al2O3 to produce a mixture of pentenoic acid isomers, (ii) carbonylation of the pentenoic acids to AA with an in situ prepared palladium catalyst, and (iii) precipitation of AA and recycling of the catalyst together with the unreacted pentenoic acid isomers to the second step (Scheme 13).158,159 In addition to unreacted GVL, AA was the only product that could be detected by 13C NMR and GC analysis when reacting the mixture of pentenoic acids present in the distillate of the first step with the palladium catalyst for 5 h under 60 bar of CO at 105 °C. Yields of 22–48% were obtained after collection of crude AA fractions by, respectively, crystallisation, filtration, washing with ethyl acetate and drying under vacuum. The selective formation of AA was tentatively attributed to the rapid equilibrium between the pentenoic acid isomers and the difference in carbonylation rate depending on the position of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.208 Indeed, 4-pentenoic acid showed a higher reactivity than the internal 2- and 3-isomers. It follows that optimization studies should be geared at improving the catalytic isomerization of pentenoic acids, alongside further efforts to effect the ring-opening of GVL during reactive distillation. To aid advances in this direction, we need more information about the kinetics and the thermodynamic constraints of the process.
C double bond.208 Indeed, 4-pentenoic acid showed a higher reactivity than the internal 2- and 3-isomers. It follows that optimization studies should be geared at improving the catalytic isomerization of pentenoic acids, alongside further efforts to effect the ring-opening of GVL during reactive distillation. To aid advances in this direction, we need more information about the kinetics and the thermodynamic constraints of the process.
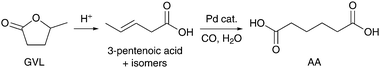 | ||
| Scheme 13 Catalytic conversion of GVL to AA via pentenoic acid.209 | ||
Analogous carbonylation processes of pentenoic acid and methyl pentenoate isomers with gaseous CO have been performed by using Pd catalysts with bidentate diphosphine ligands.210–212 As an alternative to the rather conventional gas-phase approaches,213,214 Lange et al. previously demonstrated the transesterification of GVL to methyl pentenoate under catalytic distillation conditions (Scheme 14).215,216 Key to the success of this approach was the large difference in boiling point between GVL (207 °C) and methyl pentenoate (127 °C). The reversible reaction was performed by feeding methanol into a distillation flask charged with GVL and para-toluene sulfonic acid at 200 °C. The yield of methyl pentenoate collected over consecutive distillation fractions was found to increase nearly linearly as a function of time, achieving a maximum value of 98% after completion of the reaction. Various isomers of methyl pentenoate could be detected in the distillate while the only byproducts analyzed by GC-MC were traces of pentenoic acid. The general applicability of the catalytic distillation method was shown by the transesterification of δ-hexanolactone (DHL) instead of GVL and by the substitution of methanol for higher alcohols.
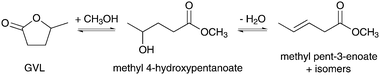 | ||
| Scheme 14 Catalytic transesterification of GVL to methyl pentenoate.215 | ||
6.4 Conversion of compounds representative of lignin and lignin-derived bio-oils
Finally, compounds representative of lignin and lignin-derived bio-oils could be exploited as another potential feedstock for AA production (Fig. 4). The aforementioned biocatalytic pathways starting from catechol or phenol provide the first opportunity in this context.217 Obviously, the catalytic conversion of phenol and substituted phenols (e.g., guaiacol or anisole)218–221 to one of the oxidation substrates discussed in Sections 2 to 5 is an alternative attractive route that could stimulate collective thinking of innovative catalytic cascade reactions. | ||
| Fig. 4 Compounds representative of lignin and lignin-derived bio-oils that could potentially be used in the catalytic production of AA. | ||
This concept can be reconciled with the substantial progress that has recently been made in the one-step hydrogenation of phenol to cyclohexanone using cooperative catalysis. Selective phenol hydrogenation at elevated conversion is complicated because the cyclohexanone product is prone to overreduction to cyclohexanol under the same conditions. However, investigations by Liu et al. revealed that this reaction can be elegantly promoted by using a synergistic combination of nanoparticulate Pd catalysts (Pd/C, Pd/Al2O3 or Pd/NaY zeolite) and Lewis acids such as AlCl3.222 Nearly quantitative conversions could be achieved within 7 h of reaction in a dichloromethane solvent at 10 bar of H2 and temperatures as low as 50 °C. Preliminary kinetic and Fourier transform infrared spectroscopic studies support the hypothesis that the Lewis acid sequentially facilitates the hydrogenation of phenol by increasing its nucleophilicity, and then inhibits the further hydrogenation of cyclohexanone (Scheme 15). Evidence was presented that coordination of the Lewis basic carbonyl oxygen to the Lewis acid is necessary to suppress the formation of cyclohexanol. Concomitant with the development of this dual catalyst system, Liu et al. showed that dichloromethane can be successfully replaced by using compressed CO2 as the reaction solvent. The facile separation of CO2, product and catalyst, as well as the proven efficiency in catalyst reuse, means that the process might be adapted to a continuous flow system, which constitutes a crucial step towards an eventual scale-up in the future.
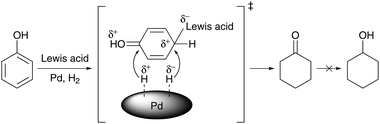 | ||
| Scheme 15 Proposed mechanism for the hydrogenation of phenol to cyclohexanone over a dual supported Pd-Lewis acid catalyst.222 | ||
7. Conclusions and outlook
Whereas the traditional industrial route involves oxidation of a mixture of cyclohexanol and cyclohexanone with nitric acid, a new generation of substrates has emerged in the past decade as an attractive resource for the future production of adipic acid. Examples discussed in this review are the selective oxidation of cyclohexene, cyclohexane, cyclohexanone or n-hexane with either hydrogen peroxide or oxygen. Important accomplishments in this research area have been achieved by the implementation of advanced process and catalyst design principles, including the use of biphasic catalytic systems, the application of continuous-flow techniques and compartmentalization by membrane reactors, the synthesis of catalytic systems that mimic natural enzymes, and the rational design of regioselective molecular sieve catalysts.To accelerate the design and development of more efficient catalysts, future research efforts should be prioritized into characterizing the nature of active sites within the catalytic systems and developing a deeper understanding of radical-based oxidation mechanisms. Equally beneficial would be gaining further insights into the underlying causes of catalyst deactivation and focusing efforts toward improving oxidant efficiency for hydrogen peroxide-mediated reactions. A pertinent question still remains unanswered regarding which of the next-generation oxidation processes has the potential to be competitive in replacing or supplementing the current industrial process. A systems-based technical and economic evaluation of all candidates based on their catalytic performance, substrate availability, catalyst stability and recyclability, oxidant efficiency and industrial feasibility is desirable to identify the most promising prospective alternative resource.
The production of adipic acid from renewable resources, particularly lignocellulosic biomass-derived chemicals, could offer an even more sustainable option. Recent breakthroughs have been made by industry and academia in the development of bio- and chemocatalytic routes for AA synthesis from biorefinery building blocks such as 5-hydroxymethylfurfural, D-glucose, γ-valerolactone and compounds representative of lignin and lignin-derived bio-oils. The successful conversion of biomass-derived oxygenates into targeted molecules will hinge on the proper design of catalysts that effectively realize catalytic cooperativity in one-pot and cascade reactions. As expertise in the field increases and new generations of catalysts and solvents continue to be designed and implemented, this research area will undoubtedly be dramatically extended in the near future.
Acknowledgements
S.V.d.V. thanks the Research Foundation – Flanders (FWO) for a postdoctoral fellowship and acknowledges support from the Belgian American Educational Foundation (BAEF), the “Plateforme pour l'Éducation et le Talent” and the Fulbright-Hays Commission for Educational Exchange between the United States and Belgium.Notes and references
- Adipic Acid: A Global Strategic Business Report, 2012, Global Industry Analysts, Inc., USA. Available electronically at http://www.strategyr.com/adipic_acid_market_report.asp.
- A. Castellan, J. C. J. Bart and S. Cavallaro, Catal. Today, 1991, 9, 237–254 CrossRef CAS
.
- I. Hermans, P. A. Jacobs and J. Peeters, Chem.–Eur. J., 2006, 12, 4229–4240 CrossRef CAS
.
-
F. Cavani and S. Alini, in Sustainable Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2009, pp. 367–425 Search PubMed
.
- V. Hessel, I. Vural Gürsel, Q. Wang, T. Noël and J. Lang, Chem. Eng. Technol., 2012, 35, 1184–1204 CrossRef CAS
.
- I. Vural Gürsel, Q. Wang, T. Noël and V. Hessel, Chem. Eng. Trans., 2012, 29, 565–570 Search PubMed
.
- I. Vural Gürsel, Q. Wang, T. Noël and V. Hessel, presented in part at the 15th Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction, Prague, August 2012.
- C. Aellig, C. Girard and I. Hermans, Angew. Chem., Int. Ed., 2011, 50, 12355–12360 CrossRef CAS
.
- C. Aellig, U. Neuenschwander and I. Hermans, ChemCatChem, 2012, 4, 525–529 CrossRef CAS
.
- C. Aellig, D. Scholz and I. Hermans, ChemSusChem, 2012, 5, 1732–1736 CrossRef CAS
.
- R. A. Reimer, C. S. Slaten, M. Seapan, M. W. Lower and P. E. Tomlinson, Environ. Prog., 1994, 13, 134–137 CrossRef CAS
.
- F. Cavani and J. H. Teles, ChemSusChem, 2009, 2, 508–534 CrossRef CAS
.
-
J. H. Teles, B. Rossler, R. Pinkos, T. Genger and T. Preiss, US Pat., 2008/0275276 A1, BASF SE, 2008 Search PubMed
.
-
J. H. Teles, B. Röβler-Feigel, A. Hauk, A. Meier, C. Müller, M. Schelper, T. Kirchner, S. Szeschkus, R. Pinkos and G.-D. Tebben, US Pat., 8,188,320, BASF SE, 2012 Search PubMed
.
- V. N. Parmon, G. I. Panov, A. Uriarte and A. S. Noskov, Catal. Today, 2005, 100, 115–131 CrossRef CAS
.
- P. Notté, Top. Catal., 2000, 13, 387–394 CrossRef
.
- P. Kubánek, B. Wichterlová and Z. Sobalik, J. Catal., 2002, 211, 109–118 Search PubMed
.
-
S. Picataggio and T. Beardslee, US Pat., 2012/0021474 A1, Verdezyne, Inc., 2012 Search PubMed
.
- T. Beardslee and S. Picataggio, Lipid Technol., 2012, 24, 223–225 CrossRef CAS
.
-
P. C. Raemakers-Franken, M. Schürmann, A. C. Trefzer and S. M. A. De Wildeman, US Pat., 2012/0028320 A1, DSM IP Assets B.V., 2012 Search PubMed
.
- R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675–5732 CrossRef CAS
.
- S. E. Smith, T. Rosendahl and P. Hofmann, Organometallics, 2011, 30, 3643–3651 CrossRef CAS
.
-
C. D. Frohning, C. W. Kohlpaintner, M. Gauß, A. Seidel, P. Torrence, P. Heymanns, A. Höhn, M. Beller, J. F. Knifton, A. Klausener, J. D. Jentsch and A. M. Tafesh, in Applied Homogeneous Catalysis with Organometallic Compounds, Wiley-VCH Verlag GmbH, 2008, pp. 27–200 Search PubMed
.
-
A. S. C. Chan and D. E. Morris, US Pat., 4,633,015, Monsanto Company, 1986 Search PubMed
.
- W. Wang, H. Liu, G. Ding, P. Zhang, T. Wu, T. Jiang and B. Han, ChemCatChem, 2012, 4, 1836–1843 CrossRef CAS
.
- F. Schwab, M. Lucas and P. Claus, Angew. Chem., Int. Ed., 2011, 50, 10453–10456 CrossRef CAS
.
- H. Liu, T. Jiang, B. Han, S. Liang, W. Wang, T. Wu and G. Yang, Green Chem., 2011, 13, 1106–1109 RSC
.
- M. Jin and Z.-M. Cheng, Catal. Lett., 2009, 131, 266–278 CrossRef CAS
.
- F. Patcas and F. C. Patcas, Catal. Today, 2006, 117, 253–258 CrossRef CAS
.
- H. Zhu, Q. Ge, W. Li, X. Liu and H. Xu, Catal. Lett., 2005, 105, 29–33 CrossRef CAS
.
- F. Cavani, J. Chem. Technol. Biotechnol., 2010, 85, 1175–1183 CrossRef CAS
.
- J. M. Thomas and R. Raja, Annu. Rev. Mater. Res., 2005, 315–350 CrossRef CAS
.
- K. Sato, M. Aoki and R. Noyori, Science, 1998, 281, 1646–1647 CrossRef CAS
.
- R. Noyori, M. Aoki and K. Sato, Chem. Commun., 2003, 1977–1986 RSC
.
- Y. Deng, Z. Ma, K. Wang and J. Chen, Green Chem., 1999, 1, 275–276 RSC
.
- P. Jin, D. Wei, Y. Wen, M. Luo, X. Wang and M. Tang, J. Mol. Struct., 2011, 992, 19–26 CrossRef CAS
.
- S. E. Jacobson, D. A. Muccigrosso and F. Mares, J. Org. Chem., 1979, 44, 921–924 CrossRef CAS
.
- Z. Bohström, I. Rico-Lattes and K. Holmberg, Green Chem., 2010, 12, 1861–1869 RSC
.
- G. Lesage, I. Q. Penate, P. Cognet and M. Poux, Int. J. Chem. React. Eng., 2012, 10, 1–18 Search PubMed
.
- P. Blach, Z. Böstrom, S. Franceschi-Messant, A. Lattes, E. Perez and I. Rico-Lattes, Tetrahedron, 2010, 66, 7124–7128 CrossRef CAS
.
- C.-Y. Cheng, K.-J. Lin, M. R. Prasad, S.-J. Fu, S.-Y. Chang, S.-G. Shyu, H.-S. Sheu, C.-H. Chen, C.-H. Chuang and M.-T. Lin, Catal. Commun., 2007, 8, 1060–1064 CrossRef CAS
.
- W. Zhu, H. Li, X. He, Q. Zhang, H. Shu and Y. Yan, Catal. Commun., 2008, 9, 551–555 CrossRef CAS
.
- M. Vafaeezadeh, M. M. Hashemi and M. Shakourian-Fard, Catal. Commun., 2012, 26, 54–57 CrossRef CAS
.
- H. Li, W. Zhu, X. He, Q. Zhang, J. Pan and Y. Yan, React. Kinet. Catal. Lett., 2007, 92, 319–327 CrossRef CAS
.
- K. Fujitani, T. Mizutani, T. Oida and T. Kawase, J. Oleo Sci., 2009, 58, 37–42 CrossRef CAS
.
- P. Jin, Z. Zhao, Z. Dai, D. Wei, M. Tang and X. Wang, Catal. Today, 2011, 175, 619–624 CrossRef CAS
.
- H. Wei, H. Li, Y. Liu, P. Jin, X. Wang and B. Li, ACS Appl. Mater. Interfaces, 2012, 4, 4106–4112 CAS
.
- S. O. Lee, R. Raja, K. D. M. Harris, J. M. Thomas, B. F. G. Johnson and G. Sankar, Angew. Chem., Int. Ed., 2003, 42, 1520–1523 CrossRef CAS
.
- G. Lapisardi, F. Chiker, F. Launay, J. P. Nogier and J. L. Bonardet, Microporous Mesoporous Mater., 2005, 78, 289–295 CrossRef CAS
.
- G. Lapisardi, F. Chiker, F. Launay, J. P. Nogier and J. L. Bonardet, Catal. Commun., 2004, 5, 277–281 CrossRef CAS
.
-
P. P. Knops-Gerrits, F. Thibault-Starzyk and P. A. Jacobs, in Stud. Surf. Sci. Catal., ed. H. G. K. H. P. J. Weitkamp and W. Hölderich, Elsevier, 1994, pp. 1411–1418 Search PubMed
.
- M. Guidotti, E. Gavrilova, A. Galarneau, B. Coq, R. Psaro and N. Ravasio, Green Chem., 2011, 13, 1806–1811 RSC
.
- M. Guidotti, C. Pirovano, N. Ravasio, B. Lazaro, J. M. Fraile, J. A. Mayoral, B. Coq and A. Galarneau, Green Chem., 2009, 11, 1421–1427 RSC
.
- J. M. Fraile, J. I. García, J. A. Mayoral and E. Vispe, J. Catal., 2001, 204, 146–156 CrossRef CAS
.
- O. A. Kholdeeva, M. S. Mel'gunov, A. N. Shmakov, N. N. Trukhan, V. V. Kriventsov, V. I. Zaikovskii, M. E. Malyshev and V. N. Romannikov, Catal. Today, 2004, 91–92, 205–209 CrossRef CAS
.
- M. N. Timofeeva, O. A. Kholdeeva, S. H. Jhung and J. S. Chang, Appl. Catal., A, 2008, 345, 195–200 CrossRef CAS
.
- J. Freitag, M. Nuchter and B. Ondruschka, Green Chem., 2003, 5, 291–295 RSC
.
- M. G. Buonomenna, G. Golemme, M. P. De Santo and E. Drioli, Org. Process Res. Dev., 2009, 14, 252–258 CrossRef
.
- Y. Wen, X. Wang, H. Wei, B. Li, P. Jin and L. Li, Green Chem., 2012, 14, 2868–2875 RSC
.
- Q. Wang, I. Vural - Gursel, M. Shimoda, M. Shang, V. Hessel and S. Ookawara, presented in part at the 22nd International Symposium on Chemical Reaction and Engineering, Maastricht, September 2012.
- T. Iwahama, K. Syojyo, S. Sakaguchi and Y. Ishii, Org. Process Res. Dev., 1998, 2, 255–260 CrossRef CAS
.
- I. Belkhir, A. Germain, F. Fajula and E. Fache, J. Chem. Soc., Faraday Trans., 1998, 94, 1761–1764 RSC
.
- H. C. Shen and H. S. Weng, Ind. Eng. Chem. Res., 1988, 27, 2254–2260 CrossRef CAS
.
- D. G. Rao and R. S. Tirukkoyilur, Ind. Eng. Chem. Process Des. Dev., 1986, 25, 299–304 CAS
.
- D. G. Rae and T. S. Raghunathan, J. Chem. Technol. Biotechnol., 1984, 34, 381–386 Search PubMed
.
- D. Bonnet, T. Ireland, E. Fache and J.-P. Simonato, Green Chem., 2006, 8, 556–559 RSC
.
- Y.-J. Xu, P. Landon, D. Enache, A. F. Carley, M. W. Roberts and G. J. Hutchings, Catal. Lett., 2005, 101, 175–179 CrossRef CAS
.
- B. P. C. Hereijgers and B. M. Weckhuysen, J. Catal., 2010, 270, 16–25 CrossRef CAS
.
- L.-X. Xu, C.-H. He, M.-Q. Zhu and S. Fang, Catal. Lett., 2007, 114, 202–205 CrossRef CAS
.
- L.-X. Xu, C.-H. He, M.-Q. Zhu, K.-J. Wu and Y.-L. Lai, Catal. Lett., 2007, 118, 248–253 CrossRef CAS
.
- G. Lü, R. Zhao, G. Qian, Y. Qi, X. Wang and J. Suo, Catal. Lett., 2004, 97, 115–118 CrossRef
.
- L. Li, C. Jin, X. Wang, W. Ji, Y. Pan, T. van der Knaap, R. van der Stoel and C. Au, Catal. Lett., 2009, 129, 303–311 CrossRef CAS
.
- G. Hutchings, S. Carrettin, P. Landon, J. Edwards, D. Enache, D. Knight, Y.-J. Xu and A. Carley, Top. Catal., 2006, 38, 223–230 CrossRef CAS
.
- A. Alshammari, A. Koeckritz, V. N. Kalevaru, A. Bagabas and A. Martin, ChemCatChem, 2012, 4, 1330–1336 CrossRef CAS
.
-
A. Alshammari, N. V. Kalevaru, A. Koeckritz and A. Martin, US Pat., 2012/0095258 A1, King Abdulaziz City for Science and Technology, 2012 Search PubMed
.
- H. Yu, F. Peng, J. Tan, X. Hu, H. Wang, J. Yang and W. Zheng, Angew. Chem., Int. Ed., 2011, 50, 3978–3982 CrossRef CAS
.
- I. Hermans, P. A. Jacobs and J. Peeters, J. Mol. Catal. A: Chem., 2006, 251, 221–228 CrossRef CAS
.
- I. Hermans, T. L. Nguyen, P. A. Jacobs and J. Peeters, ChemPhysChem, 2005, 6, 637–645 CrossRef CAS
.
- X. Yang, H. Yu, F. Peng and H. Wang, ChemSusChem, 2012, 5, 1213–1217 CrossRef CAS
.
- R. F. Parton, G. J. Peere, P. E. Neys, P. A. Jacobs, R. Claessens and G. V. Baron, J. Mol. Catal. A: Chem., 1996, 113, 445–454 CrossRef CAS
.
-
F. Thibault-Starzyk, R. F. Parton and P. A. Jacobs, in Stud. Surf. Sci. Catal., ed. H. G. K. H. P. J. Weitkamp and W. Hölderich, Elsevier, 1994, pp. 1419–1424 Search PubMed
.
- R. F. Parton, I. F. J. Vankelecom, M. J. A. Casselman, C. P. Bezoukhanova, J. B. Uytterhoeven and P. A. Jacobs, Nature, 1994, 370, 541–544 CrossRef CAS
.
- Y. Yuan, H. Ji, Y. Chen, Y. Han, X. Song, Y. She and R. Zhong, Org. Process Res. Dev., 2004, 8, 418–420 CrossRef CAS
.
- H. Noack, V. Georgiev, M. R. A. Blomberg, P. E. M. Siegbahn and A. J. Johansson, Inorg. Chem., 2011, 50, 1194–1202 CrossRef CAS
.
- P. S. Starcher and B. Phillips, J. Am. Chem. Soc., 1958, 80, 4079–4082 CrossRef CAS
.
- I. Hermans, E. Spier, U. Neuenschwander, N. Turrà and A. Baiker, Top. Catal., 2009, 52, 1162–1174 CrossRef CAS
.
- M. Besson, A. Blackburn, P. Gallezot, O. Kozynchenko, A. Pigamo and S. Tennison, Top. Catal., 2000, 13, 253–257 CrossRef CAS
.
- A. Pigamo, M. Besson, B. Blanc, P. Gallezot, A. Blackburn, O. Kozynchenko, S. Tennison, E. Crezee and F. Kapteijn, Carbon, 2002, 40, 1267–1278 CrossRef CAS
.
- M. Besson, F. Gauthard, B. Horvath and P. Gallezot, J. Phys. Chem. B, 2004, 109, 2461–2467 CrossRef
.
- E. Crezee, A. Barendregt, F. Kapteijn and J. A. Moulijn, Catal. Today, 2001, 69, 283–290 CrossRef CAS
.
- A. Shimizu, K. Tanaka, H. Ogawa, Y. Matsuoka, M. Fujimori, Y. Nagamori, H. Hamachi and K. Kimura, Bull. Chem. Soc. Jpn., 2003, 76, 1993–2001 CrossRef CAS
.
- S. A. Chavan, D. Srinivas and P. Ratnasamy, J. Catal., 2002, 212, 39–45 CrossRef CAS
.
- F. Cavani, L. Ferroni, A. Frattini, C. Lucarelli, A. Mazzini, K. Raabova, S. Alini, P. Accorinti and P. Babini, Appl. Catal., A, 2011, 391, 118–124 CrossRef CAS
.
- S. Kirumakki, S. Samarajeewa, R. Harwell, A. Mukherjee, R. H. Herber and A. Clearfield, Chem. Commun., 2008, 5556–5558 RSC
.
- A. Corma, L. T. Nemeth, M. Renz and S. Valencia, Nature, 2001, 412, 423–425 CrossRef CAS
.
- A. Dutta, M. Pramanik, A. K. Patra, M. Nandi, H. Uyama and A. Bhaumik, Chem. Commun., 2012, 48, 6738–6740 RSC
.
- N. K. Mal, S. Ichikawa and M. Fujiwara, Chem. Commun., 2002, 112–113 RSC
.
- Y. Usui and K. Sato, Green Chem., 2003, 5, 373–375 RSC
.
- R. D. Bach, J. Org. Chem., 2012, 77, 6801–6815 CrossRef CAS
.
- M. Boronat, A. Corma, M. Renz, G. Sastre and P. M. Viruela, Chem.–Eur. J., 2005, 11, 6905–6915 CrossRef CAS
.
- F. Cavani, K. Raabova, F. Bigi and C. Quarantelli, Chem.–Eur. J., 2010, 16, 12962–12969 CrossRef CAS
.
- A. O. Terent'ev, M. M. Platonov, A. S. Kashin and G. I. Nikishin, Tetrahedron, 2008, 64, 7944–7948 CrossRef CAS
.
- A. O. Terent'ev, M. M. Platonov, Y. N. Ogibin and G. I. Nikishin, Synth. Commun., 2007, 37, 1281–1287 CrossRef CAS
.
- A. Terent’ev, M. Platonov and A. Kutkin, Cent. Eur. J. Chem., 2006, 4, 207–215 CrossRef
.
- R. Raja, G. Sankar and J. M. Thomas, Angew. Chem., Int. Ed., 2000, 39, 2313–2316 CrossRef CAS
.
- R. Raja and J. M. Thomas, J. Mol. Catal. A: Chem., 2002, 181, 3–14 CrossRef CAS
.
- J. M. Thomas, R. Raja, G. Sankar and R. G. Bell, Acc. Chem. Res., 2001, 34, 191–200 CrossRef CAS
.
- J. M. Thomas, R. Raja, G. Sankar and R. G. Bell, Nature, 1999, 398, 227–230 CrossRef CAS
.
- G. Sankar, R. Raja and J. Thomas, Catal. Lett., 1998, 55, 15–23 CrossRef CAS
.
- M. Hartmann and S. Ernst, Angew. Chem., Int. Ed., 2000, 39, 888–890 CrossRef CAS
.
- R. Raja, G. Sankar and J. Meurig Thomas, Chem. Commun., 1999, 829–830 RSC
.
- R. Raja, J. Meurig Thomas and G. Sankar, Chem. Commun., 1999, 525–526 RSC
.
- B. Moden, B. Z. Zhan, J. Dakka, J. G. Santiesteban and E. Iglesia, J. Catal., 2006, 239, 390–401 CrossRef CAS
.
- R. Raja, J. M. Thomas, M. C. Xu, K. D. M. Harris, M. Greenhill-Hooper and K. Quill, Chem. Commun., 2006, 448–450 RSC
.
- M. Dugal, G. Sankar, R. Raja and J. M. Thomas, Angew. Chem., Int. Ed., 2000, 39, 2310–2313 CrossRef CAS
.
- R. Raja, G. Sankar and J. M. Thomas, J. Am. Chem. Soc., 1999, 121, 11926–11927 CrossRef CAS
.
- D. L. Vanoppen, D. E. De Vos, M. J. Genet, P. G. Rouxhet and P. A. Jacobs, Angew. Chem., 1995, 107, 637–639 CrossRef
.
- N. R. Shiju, S. Fiddy, O. Sonntag, M. Stockenhuber and G. Sankar, Chem. Commun., 2006, 4955–4957 RSC
.
- B. Moden, L. Oliviero, J. Dakka, J. G. Santiesteban and E. Iglesia, J. Phys. Chem. B, 2004, 108, 5552–5563 CrossRef CAS
.
- L. Gómez-Hortigüela, F. Corà, G. Sankar, C. M. Zicovich-Wilson and C. R. A. Catlow, Chem.–Eur. J., 2010, 16, 13638–13645 CrossRef
.
- L. Gómez-Hortigüela, F. Corà and C. R. A. Catlow, ACS Catal., 2010, 1, 18–28 CrossRef
.
- L. Gómez-Hortigüela, F. Corà and C. R. A. Catlow, ACS Catal., 2011, 1, 945–955 CrossRef
.
- L. Gómez-Hortigüela, F. Corà and C. R. A. Catlow, ACS Catal., 2011, 1, 1487–1497 CrossRef
.
- L. Gómez-Hortigüela, F. Corà and C. R. A. Catlow, ACS Catal., 2011, 1, 1475–1486 CrossRef
.
- H. Lü, W. Ren, P. Liu, S. Qi, W. Wang, Y. Feng, F. Sun and Y. Wang, Appl. Catal., A, 2012, 441–442, 136–141 CrossRef
.
- J. Hilgert, N. Meine, R. Rinaldi and F. Schüth, Energy Environ. Sci., 2013, 6, 92–96 CAS
.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564–2601 CrossRef CAS
.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC
.
- S. Van de Vyver, J. Geboers, W. Schutyser, M. Dusselier, P. Eloy, E. Dornez, J. W. Seo, C. M. Courtin, E. M. Gaigneaux, P. A. Jacobs and B. F. Sels, ChemSusChem, 2012, 5, 1549–1558 CrossRef CAS
.
- S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef CAS
.
- J. A. Geboers, S. Van de Vyver, R. Ooms, B. Op de Beeck, P. A. Jacobs and B. F. Sels, Catal. Sci. Technol., 2011, 1, 714–726 Search PubMed
.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520–540 RSC
.
- E. Nikolla, Y. Román-Leshkov, M. Moliner and M. E. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS
.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS
.
- B. H. Shanks, Ind. Eng. Chem. Res., 2010, 49, 10212–10217 CrossRef CAS
.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610–626 CAS
.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS
.
- J. M. R. Gallo, D. M. Alonso, M. A. Mellmer and J. A. Dumesic, Green Chem., 2013, 15, 85–90 RSC
.
- C. Aellig and I. Hermans, ChemSusChem, 2012, 5, 1737–1742 CrossRef CAS
.
- J. Wang, J. Ren, X. Liu, J. Xi, Q. Xia, Y. Zu, G. Lu and Y. Wang, Green Chem., 2012, 14, 2506–2512 RSC
.
- T. Wang, Y. Pagán-Torres, E. Combs, J. Dumesic and B. Shanks, Top. Catal., 2012, 55, 657–662 CrossRef CAS
.
- Y. J. Pagán-Torres, T. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef
.
- T. Deng, X. Cui, Y. Qi, Y. Wang, X. Hou and Y. Zhu, Chem. Commun., 2012, 48, 5494–5496 RSC
.
- S. Zhao, M. Cheng, J. Li, J. Tian and X. Wang, Chem. Commun., 2011, 47, 2176–2178 RSC
.
- A. Crisci, M. Tucker, J. Dumesic and S. Scott, Top. Catal., 2010, 53, 1185–1192 CrossRef CAS
.
- J. N. Chheda, Y. Roman-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC
.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef
.
-
M. Faber, US Pat., 4,400,468, Hydrocarbon Research Inc., 1983 Search PubMed
.
- R. Alamillo, M. Tucker, M. Chia, Y. Pagan-Torres and J. Dumesic, Green Chem., 2012, 14, 1413–1419 RSC
.
- T. Buntara, S. Noel, P. H. Phua, I. Melián-Cabrera, J. G. de Vries and H. J. Heeres, Angew. Chem., Int. Ed., 2011, 50, 7083–7087 CrossRef CAS
.
- T. Buntara, S. Noel, P. Phua, I. Melián-Cabrera, J. Vries and H. Heeres, Top. Catal., 2012, 55, 612–619 CrossRef CAS
.
- M. Chia, Y. J. Pagán-Torres, D. Hibbitts, Q. Tan, H. N. Pham, A. K. Datye, M. Neurock, R. J. Davis and J. A. Dumesic, J. Am. Chem. Soc., 2011, 133, 12675–12689 CrossRef CAS
.
- K. Chen, S. Koso, T. Kubota, Y. Nakagawa and K. Tomishige, ChemCatChem, 2010, 2, 547–555 CrossRef CAS
.
-
T. R. Boussie, E. L. Dias, Z. M. Fresco and V. J. Murphy, US Pat., 2010/0317822 A1, Rennovia, Inc., 2010 Search PubMed
.
- N. K. Gupta, S. Nishimura, A. Takagaki and K. Ebitani, Green Chem., 2011, 13, 824–827 RSC
.
- W. Partenheimer and V. V. Grushin, Adv. Synth. Catal., 2001, 343, 102–111 CrossRef CAS
.
- P. Verdeguer, N. Merat and A. Gaset, J. Mol. Catal., 1993, 85, 327–344 CrossRef CAS
.
- M. L. Ribeiro and U. Schuchardt, Catal. Commun., 2003, 4, 83–86 CrossRef CAS
.
- M. Kröger, U. Prüße and K.-D. Vorlop, Top. Catal., 2000, 13, 237–242 CrossRef
.
- O. Casanova, S. Iborra and A. Corma, ChemSusChem, 2009, 2, 1138–1144 CrossRef CAS
.
-
T. R. Boussie, E. L. Dias, Z. M. Fresco, V. J. Murphy, J. Shoemaker, R. Archer and H. Jiang, US Pat., 2010/0317823 A1, Rennovia, Inc., 2010 Search PubMed
.
- Based on data and information from ICIS Chemical Business. Available electronically at http://www.icis.com.
- A. Abbadi and H. van Bekkum, J. Mol. Catal. A: Chem., 1995, 97, 111–118 CrossRef CAS
.
- P. J. M. Dijkgraaf, H. A. M. Duisters, B. F. M. Kuster and K. van der Wiele, J. Catal., 1988, 112, 337–344 CrossRef CAS
.
- J. M. H. Dirkx and H. S. van der Baan, J. Catal., 1981, 67, 1–13 CrossRef CAS
.
- J. M. H. Dirkx, H. S. van der Baan and J. M. A. J. J. van den Broek, Carbohydr. Res., 1977, 59, 63–72 CrossRef CAS
.
- W. Niu, K. M. Draths and J. W. Frost, Biotechnol. Prog., 2002, 18, 201–211 CrossRef CAS
.
- K. M. Draths and J. W. Frost, J. Am. Chem. Soc., 1994, 116, 399–400 CrossRef CAS
.
- J. M. Thomas, R. Raja, B. F. G. Johnson, T. J. O’Connell, G. Sankar and T. Khimyak, Chem. Commun., 2003, 1126–1127 RSC
.
- X. She, H. M. Brown, X. Zhang, B. K. Ahring and Y. Wang, ChemSusChem, 2011, 4, 1071–1073 CrossRef CAS
.
- Z. J. Reitman, B. D. Choi, I. Spasojevic, D. D. Bigner, J. H. Sampson and H. Yan, Nat. Chem. Biol., 2012, 8, 887–889 CrossRef CAS
.
-
A. P. Burgard, P. Pharkya and R. E. Osterhout, US Pat., 8,088,607 B2, Genomatica, Inc., 2012 Search PubMed
.
- A. Parthasarathy, A. J. Pierik, J. r. Kahnt, O. Zelder and W. Buckel, Biochemistry, 2011, 50, 3540–3550 CrossRef CAS
.
- T. Polen, M. Spelberg and M. Bott, J. Biotechnol, 2012 DOI:10.1016/j.jbiotec.2012.1007.1008
.
- W. R. H. Wright and R. Palkovits, ChemSusChem, 2012, 5, 1657–1667 CrossRef CAS
.
- L. Qi and I. T. Horváth, ACS Catal., 2012, 2, 2247–2249 CrossRef CAS
.
- J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS
.
- M. G. Al-Shaal, W. R. H. Wright and R. Palkovits, Green Chem., 2012, 14, 1260–1263 RSC
.
- A. M. R. Galletti, C. Antonetti, V. De Luise and M. Martinelli, Green Chem., 2012, 14, 688–694 RSC
.
- W. Li, J.-H. Xie, H. Lin and Q.-L. Zhou, Green Chem., 2012, 14, 2388–2390 RSC
.
- S. G. Wettstein, J. Q. Bond, D. M. Alonso, H. N. Pham, A. K. Datye and J. A. Dumesic, Appl. Catal., B, 2012, 117–118, 321–329 CrossRef CAS
.
- J. M. Tukacs, D. Kiraly, A. Stradi, G. Novodarszki, Z. Eke, G. Dibo, T. Kegl and L. T. Mika, Green Chem., 2012, 14, 2057–2065 RSC
.
- A. M. Hengne and C. V. Rode, Green Chem., 2012, 14, 1064–1072 RSC
.
- M. Chia and J. A. Dumesic, Chem. Commun., 2011, 47, 12233–12235 RSC
.
- E. I. Gürbüz, D. M. Alonso, J. Q. Bond and J. A. Dumesic, ChemSusChem, 2011, 4, 357–361 CrossRef
.
- D. J. Braden, C. A. Henao, J. Heltzel, C. C. Maravelias and J. A. Dumesic, Green Chem., 2011, 13, 1755–1765 RSC
.
- M. Chalid, A. A. Broekhuis and H. J. Heeres, J. Mol. Catal. A: Chem., 2011, 341, 14–21 CrossRef CAS
.
- D. Martin Alonso, J. Gallo, M. Mellmer, S. Wettstein and J. Dumesic, Catal. Sci. Technol., 2013 10.1039/C1032CY20689G
.
- R. Weingarten, W. C. Conner and G. W. Huber, Energy Environ. Sci., 2012, 5, 7559–7574 CAS
.
- S. G. Wettstein, D. M. Alonso, Y. Chong and J. A. Dumesic, Energy Environ. Sci., 2012, 5, 8199–8203 CAS
.
- Z. Sun, M. Cheng, H. Li, T. Shi, M. Yuan, X. Wang and Z. Jiang, RSC Adv., 2012, 2, 9058–9065 RSC
.
- S. Van de Vyver, S. Helsen, J. Geboers, F. Yu, J. Thomas, M. Smet, W. Dehaen, Y. Román-Leshkov, I. Hermans and B. F. Sels, ACS Catal., 2012, 2700–2704 CrossRef CAS
.
- S. Van de Vyver, J. Geboers, S. Helsen, F. Yu, J. Thomas, M. Smet, W. Dehaen and B. F. Sels, Chem. Commun., 2012, 48, 3497–3499 RSC
.
- S. Van de Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Energy Environ. Sci., 2011, 4, 3601–3610 CAS
.
- J.-P. Lange, R. Price, P. M. Ayoub, J. Louis, L. Petrus, L. Clarke and H. Gosselink, Angew. Chem., Int. Ed., 2010, 49, 4479–4483 CrossRef CAS
.
- B. Girisuta, B. Danon, R. Manurung, L. P. B. M. Janssen and H. J. Heeres, Bioresour. Technol., 2008, 99, 8367–8375 CrossRef CAS
.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Ind. Eng. Chem. Res., 2007, 46, 1696–1708 CrossRef CAS
.
- D. M. Alonso, S. G. Wettstein, M. A. Mellmer, E. I. Gurbuz and J. A. Dumesic, Energy Environ. Sci., 2013, 6, 76–80 CAS
.
- E. I. Gürbüz, S. G. Wettstein and J. A. Dumesic, ChemSusChem, 2012, 5, 383–387 CrossRef
.
- P. Azadi, R. Carrasquillo-Flores, Y. J. Pagan-Torres, E. I. Gurbuz, R. Farnood and J. A. Dumesic, Green Chem., 2012, 14, 1573–1576 RSC
.
- S. M. Sen, D. M. Alonso, S. G. Wettstein, E. I. Gurbuz, C. A. Henao, J. A. Dumesic and C. T. Maravelias, Energy Environ. Sci., 2012, 5, 9690–9697 CAS
.
-
E. M. Atadan and J. H. S. Bruner, US Pat., 5,292,944, E. I. Du Pont de Nemours and Company, 1994 Search PubMed
.
-
P. Denis, J.-M. Grosselin and F. Metz, US Pat., 5,227,522, Rhone-Poulenc Chimie, 1993 Search PubMed
.
-
J. H. S. Bruner, S. L. Lane and B. E. Murphree, US Pat., 5,710,325, E. I. Du Pont de Nemours and Company, DSM, N.V., 1998 Search PubMed
.
-
P. M. Burke, European Pat., EP0395038 (A2), E. I. Du Pont de Nemours and Company, 1990 Search PubMed
.
-
P. M. Burke, US Pat., 4,939,298, E. I. du Pont de Nemours and Company, 1990 Search PubMed
.
- J. Q. Bond, D. Martin Alonso, R. M. West and J. A. Dumesic, Langmuir, 2010, 26, 16291–16298 CrossRef CAS
.
-
P. K. Wong, C. Li, L. Stubbs, M. Van Meurs, D. G. Anak Kumbang, S. C. Y. Lim and E. Drent, International Pat., WO/2012/134397, Agency for Science, Technology and Research, 2012 Search PubMed
.
- M. van Meurs, presented in part at the 2012 TUDCE-ICES Workshop on Green Innovation for Chemical Technologies, Singapore, April, 2012.
-
J. G. de Vries, N. Sereinig, E. W. M. Van De Vondervoort and M. C. C. Janssen, International Pat., WO/2012/131028, DSM IP Assets B.V., 2012 Search PubMed
.
-
E. Drent, R. Ernst, W. W. Jager, C. A. Krom, T. M. Nisbet and J. A. M. Van Broekhoven, International Pat., WO/2006/125801, Shell Internationale Research Maatschappij B.V., 2006 Search PubMed
.
-
E. Drent and W. W. Jager, International Pat., WO0168583 (A2), Shell Internationale Research Maatschappij B.V., 2001 Search PubMed
.
-
L. E. Manzer, US Pat., WO/2004/007421 A1, EI Du Pont de Nemours and Company, 2004 Search PubMed
.
-
R. Fischer and U. Vagi, US Pat., 4,740,613, BASF Aktiengesellschaft, 1988 Search PubMed
.
- J.-P. Lange, J. Z. Vestering and R. J. Haan, Chem. Commun., 2007, 3488–3490 RSC
.
-
R. J. Haan and J.-P. Lange, US Pat., US 2007/0142664 A1, Shell Oil Company, 2007 Search PubMed
.
- J. B. J. H. van Duuren, B. Brehmer, A. E. Mars, G. Eggink, V. A. P. M. dos Santos and J. P. M. Sanders, Biotechnol. Bioeng., 2011, 108, 1298–1306 CrossRef CAS
.
- R. C. Runnebaum, T. Nimmanwudipong, D. E. Block and B. C. Gates, Catal. Sci. Technol., 2012, 2, 113–118 CAS
.
- T. Nimmanwudipong, R. Runnebaum, D. Block and B. Gates, Catal. Lett., 2011, 141, 779–783 CrossRef CAS
.
- R. Runnebaum, T. Nimmanwudipong, D. Block and B. Gates, Catal. Lett., 2011, 141, 817–820 CrossRef CAS
.
- T. Prasomsri, A. T. To, S. Crossley, W. E. Alvarez and D. E. Resasco, Appl. Catal., B, 2011, 106, 204–211 CAS
.
- H. Liu, T. Jiang, B. Han, S. Liang and Y. Zhou, Science, 2009, 326, 1250–1252 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |
