 Open Access Article
Open Access ArticleNon-canonical amino acids as a useful synthetic biological tool for lipase-catalysed reactions in hostile environments†
Carlos G.
Acevedo-Rocha‡
*ab,
Michael G.
Hoesl‡
c,
Sebastian
Nehring‡
c,
Marina
Royter
d,
Christina
Wolschner
c,
Birgit
Wiltschi
e,
Garabed
Antranikian
d and
Nediljko
Budisa
c
aMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim, Germany. E-mail: acevedor@kofo.mpg.de
bPhilipps-Universität Marburg, Fachbereich Chemie, Hans-Meerwein-Straße, 35032 Marburg, Germany. Tel: +49 (0)-6421-28-26082
cBerlin Institute of Technology, Department of Chemistry, Biocatalysis Group, Franklinstrasse 29, 10587 Berlin, Germany
dInstitute of Technical Microbiology, Hamburg University of Technology, Kasernenstrasse 12, 21073 Hamburg, Germany
eACIB, Petersgasse 14, 8010 Graz, Austria
First published on 14th February 2013
Abstract
The incorporation of several non-canonical amino acids into the Thermoanaerobacter thermohydrosulfuricus lipase confers not only activity enhancement upon treatment with organic solvents (by up to 450%) and surfactants (resp. 1630%), but also protective effects against protein reducing (resp. 140%), alkylating (resp. 160%), and denaturing (resp.190%) agents as well as inhibitors (resp. 40%). This approach offers novel chemically diversified biocatalysts for hostile environments.
Lipases are the most important biocatalysts in organic chemistry and biotechnology, but often these do not display stability towards hostile environments necessary for industrial transformations.1–3 To solve this issue, several strategies include the use of additives,3 enzyme chemical modification/immobilization,2 freeze-drying,3 or pretreatment with organic solvents.4 Directed evolution has also emerged as a reliable tool for protein engineering,5–7 including random8 or structure-guided9 approaches for improving stability of enzymes towards organic solvents10 or other hostile environments.11 From the synthetic biology point of view, however, it would be useful to introduce alternative methods. For example, the global incorporation of non-canonical amino acids (ncAAs) into proteins could deliver novel interactions by modifying residue hydropathy, surface areas, polarities, ionisation states and covalent radii in a radical manner that the standard twenty canonical amino acids cannot always achieve.12 These physical properties are important enzymatic activity determinants in apolar solvents because at lower dielectric constants proteins show stronger intra-protein electrostatic interactions and more rigid structures.13 We recently reported that ncAAs with various chemical modifications dramatically altered the functionality of a lipase from the thermostable bacterium Thermoanaerobacter thermohydrosulfuricus (TTL).14 The observed effects include among others enhanced hydrolytic activity, activation efficiency, as well as substantial changes in optimal temperature and pH values (Table S1, ESI†).
In this communication, we report the influence of various organic solvents on the hydrolytic activity of TTL congeners (these proteins originate from the same parent gene sequence, but contain a fraction of ncAAs). In addition, we also explored the effects of various metal cations, surfactants, protein reducing, alkylating and denaturing agents as well as inhibitors. Here we used the tryptophan (1) analogues 4-aminotryptophan (1a), 4-fluorotryptophan (1b), and 7-azatryptophan (1c); and we also included the previous TTL congeners,14 containing the proline (2) analogues cis-4-fluoroproline (2a), trans-4-fluoroproline (2b), cis-4-hydroxyproline (2c), and trans-4-hydroxyproline (2d); the tyrosine (3) analogues ortho-fluorotyrosine (3a) and meta-fluorotyrosine (3b); the methionine (4) analogues norleucine (4a) and azidohomoalanine (4b); and the phenylalanine (5) analogues meta-fluorophenylalanine (5a) and para-fluorophenylalanine (5b) (Scheme 1).
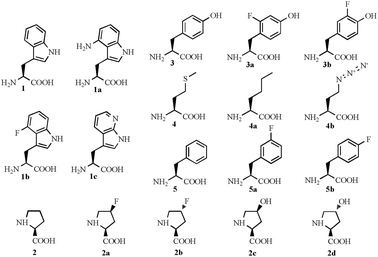 | ||
| Scheme 1 Canonical and non-canonical amino acids used. | ||
The parent TTL and thirteen TTL congeners were expressed using appropriate amino acid auxotrophic Escherichia coli strains under controlled culture conditions, followed by purification and analytical characterization to assess ncAA incorporation efficiency (see ESI† and Table S1). As noted earlier,14 TTL is a lipase that displays thermal activation, i.e., it requires heat to display a shift (Thermal Activation Factor or TAF) of 14-fold from its basal to its maximal activity (see Table S1, ESI†). Importantly, the enzymatic activation most likely depends on the opening or closing of a lid covering TTL's active site (see Fig. S1 and discussion in Table S1, ESI†). Interestingly, while TTL[4a] is the only congener that does not require activation to be as active as parent TTL, other congeners such as TTL[1b/3a] require different activation values (resp. 11/7-fold; Table S1, ESI†). Exceptionally, TTL[5a] combines a TAF of only 6-fold with a higher activity than parent TTL by 1.25-fold (Table S1, ESI†). These findings suggest that the incorporation of ncAAs may be a strategy to not only activate lipases, but also to confer them functional advantages. While currently we are trying to gain structural insight into the TTL congeners studied, we decided to gain more functional knowledge by pre-treating these with organic solvents, metal ions, surfactants, protein reducing, alkylating and denaturing agents as well as inhibitors. The congeners were thus incubated with the various substances for 1 hour at 25 °C, and the residual hydrolytic activity was then measured using p-nitrophenyl palmitate ester, as described in the ESI.†
The treatment of lipases with organic solvents both enhanced and impaired lipase activity (Table 1). In parent TTL, most solvents were deleterious (<0.8-fold), with pyridine, DMSO and toluol displaying the highest effects (0.0-fold), in contrast to the slightly positive effects of tert-butanol (1.1-fold) and hexadecane (1.3-fold; Table 1).
| a TTL congeners were incubated for 1 hour at 25 °C with 90% of organic solvent. b To correct for p-nitrophenol auto-hydrolysis due to chemical agent exposure and buffer, samples without lipase and without solvent were, respectively, included. Lipase activity was measured in triplicate for all cases at the optimal temperature and pH 8.0 as described in the ESI. To assess the sole effect of solvents, the values were normalized to 1.0 for each TTL. c Negative effects are displayed on a white background (less than 1.0), whereas neutral and positive effects are shown on light (1.0) and dark (more than 1.0) grey backgrounds, resp. DMSO: dimethyl sulfoxide; DMF: dimethylformamide; NDA: N-decyl-alcohol. |
|---|
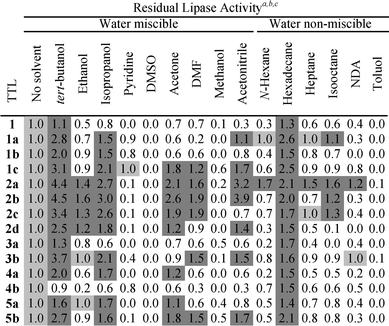
|
In the case of the 1 (Trp) congeners, noteworthy are the minimal effects on the activity of TTL[1a/1b/1c] after pyridine treatment by 0.8 to 1.0-fold, in contrast to all congeners, except for TTL[4b], also by 0.8-fold (Table 1). This suggests that the ncAAs at the two unique Trp residues, which are located close to the active site of TTL (Fig. S1, ESI†), may interact with pyridine to prevent lipase inactivation. These findings are important because pyridine is useful for the lipase-catalysed synthesis of biodegradable polymers.15 Of interest is also the finding that TTL[1c] can be more active than TTL[1a/1b] after tert-butanol, isopropanol, acetone, DMF, methanol, acetonitrile and N-decyl-alcohol treatment (Table 1). Ethanol, heptane and isooctane did not significantly alter the activity of all Trp congeners, but these were completely inhibited by DMSO and toluol (Table 1). In contrast, TTL[1a] was slightly better than TTL[1b/1c] only upon exposure to n-hexane and hexadecane, though minimally (Table 1). Interestingly, under normal conditions (without solvent treatment and taking account of TAF), TTL[1a] and TTL[1c], respectively, lost their maximal activities from 32 to 25 and 10 mU μg−1, whereas that of TTL[1b] was negligibly affected from 32 to 31 mU μg−1 (Table S1, ESI†). These findings suggest that 1b may facilitate the exposure of the active site for catalysis, a clear advantage in aqueous environments, but not in non-aqueous ones. Although 1c/1a may trade-off the activity of TTL under standard conditions, the potential advantages of using these ncAAs in organic media are evident. It is thus unadvisable to heat-activate the congeners prior to organic solvent exposure, since the likelihood of compromising their activity exists.
Regarding 2 (Pro) congeners, the fluorinated ones, TTL[2a/2b], were generally more active than the hydroxylated ones, TTL[2c/2d], upon treatment with tert-butanol, ethanol, isopropanol, acetone, DMF, acetonitrile, n-hexane, hexadecane, isooctane and n-decyl-alcohol (Table 1). Like parent TTL, all Pro congeners practically did not resist the effects of pyridine, DMSO, methanol and toluol, as no residual lipase activity was detected (Table 1). Under aqueous conditions, however, the original lipase activity was affected after maximal heat activation from 32 to 8.3/6.3/6.6/10.4 mU μg−1 in TTL[2a/2b/2c/2d], resp. (Table S1, ESI†). Although the Pro congeners were marginally active in water, the ncAAs conferred them a selective advantage upon organic media treatment, TTL[2b] being the one with the highest effect by up to 4.5-fold in tert-butanol (Table 1), an important co-solvent for the lipase-catalysed production of biodiesel.16 Another practical example is TTL[2a] that displays the highest activity after treatment with isooctane (1.6-fold), another important co-solvent for the lipase-based synthesis of biodiesel from waste oils as feedstocks.16
The most prominent feature of globally substituting the seven 3 (Tyr) residues (Fig. S1, ESI†) in TTL by fluorinated analogues is the dependence of activity on the position of the ring substitution. Under aqueous conditions, the fluorination at the meta-position (3b) markedly affected the maximal activity (3.7 mU μg−1), whereas fluorination at the ortho-position (3a) did not (30.3 mU μg−1), the latter being very close to that of parent TTL (32 mU μg−1; Table S1, ESI†). The main functional difference, under standard conditions, between TTL and TTL[3a] is a TAF of 14 and 7, resp. (Table S1, ESI†). However, TTL[3b] displayed more activity than TTL and TTL[3a] after pre-treatment with all organic solvents, except for methanol in the latter case (Table 1). Remarkably, TTL[3a] reveals exactly the same patterns as parent TTL after organic solvent treatment – activity enhancement with tert-butanol and hexadecane but various degrees of impairment with all the other solvents (Table 1). Curiously, parent TTL and TTL[3a] have the same optimal pH and temperature as well as similar maximal activity upon heat activation with different TAF values, in contrast to TTL[3b] with a 20 °C shift in optimal temperature and loss of activity under aqueous conditions (Table S1, ESI†). That TTL and TTL[3a] have the same patterns in their functional properties, while TTL[3a] and TTL[3b] exhibit opposite ones in spite of the “subtle” structural differences between 3a and 3b (ring substitution of fluorine) deserves further attention.
Regarding the 4 (Met) congeners, TTL[4a] does not require heat activation under standard conditions to show maximal activity, but TTL[4b] needs a much higher thermal activation to reach 1/3 of that of TTL[4a] and parent TTL (Table S1, ESI†). Based on these findings, and on the location of the three out of the eleven Met residues in the lid (Fig. S1, ESI†), we suggested before that the hydrophobic character of 4a may have retained the TTL's lid in an open conformation, whereas 4b, hydrophilic in nature, may have blocked lid opening.14 If this may be plausible, then TTL[4a] would have its active site more exposed and, thus, it would be more vulnerable to organic solvent treatment, in contrast to TTL[4b]. Interestingly, TTL[4a] and TTL[3b] display very low resistance to most organic solvents, similar to parent TTL, but TTL[4b] was less active in most of them, except after treatment with pyridine (Table 1). That TTL[4b] has such a low catalytic activity may be due to a complete blockage of the lid opening. However, further structural investigations are needed, to clarify this issue.
In the case of the 5 (Phe) congeners, upon maximal heat activation and under standard conditions, TTL[5a] was the most active of all lipases, whereas TTL[5b] lost 2/3 activity (Table S1, ESI†). In contrast, TTL[5b] performed better than TTL[5a] after tert-butanol, acetone, DMF, acetonitrile and hexadecane treatment, but both were equal regarding all the other solvents (Table 1). The high number of Phe residues (sixteen) in TTL might indicate a functional importance, as many are located in and near the lid as well as the active site (Fig. S1, ESI†).
All congeners were also pre-incubated with several metal ions because it is known that the latter ones can influence lipase activity.17 Unfortunately, the activity of all congeners, including parent TTL, was impaired by the treatment with all tri- and divalent cations, as well as Ag+, but not by the mono-cations K+ and Na+ (Table S2, ESI†). This could be explained by the possible interaction of the tri- and divalent ions with the C-terminal His-Tag, which can be inferred from divalent copper, known to have affinity for histidine, since non-His-tagged TTL is indifferent to CuCl2 pre-treatment,18 whereas TTL with a His-Tag lost its activity by 2/3 (Table S2, ESI†).
We also examined lipase activity of the TTL congeners upon treatment with surfactants, protein denaturing, reducing and alkylating agents as well as inhibitors (Table S3, ESI†). Of interest is the surfactant CHAPS, which enhanced the activity of almost all lipases, TTL[2d] being the most benefited by up to 16.3-fold vs. 1.7-fold of TTL. Similarly, upon treatment with the denaturant guanidium chloride TTL[1a/5a] resisted 0.7-fold vs. no activity of TTL, while the activity of TTL[1c] increased by 1.9-fold vs. 0.9-fold of TTL after urea exposure (Table S3, ESI†). Finally, the protein inhibitor pefabloc inactivated almost all congeners, except for TTL[2a/3b], which displayed a residual activity of 0.4-fold (Table S3, ESI†). Importantly, the ring substituent is critical to confer partial “immunity” as TTL[2b/3a] was completely inactivated.
In summary, the incorporation of ncAAs into this particular lipase not only enhanced its hydrolytic residual activity upon treatment with organic solvents and surfactants, but also conferred it protective effects against protein denaturing, alkylating, denaturing, and inhibitory substances. Our data not only show the potential advantages of using ncAAs in lipase-catalyzed reactions in hostile environments, but also highlight the challenges ahead to meet the required traits such as selectivity for practical biotransformations.
This work was supported by the UniCat Excellence Cluster in Berlin as well as by the BioFuture Program and the Biokatalyse 2021 cluster, both of the German Federal Ministry for Education and Research.
Notes and references
- U. T. Bornscheuer and R. J. Kazlauskas, Hydrolases in Organic Synthesis: Regio- and Stereoselective Biotransformations, Wiley-VCH, Weinheim, 2005 Search PubMed.
- K. M. Polizzi, A. S. Bommarius, J. M. Broering and J. F. Chaparro-Riggers, Curr. Opin. Chem. Biol., 2007, 11, 220–225 CrossRef CAS.
- A. L. Serdakowski and J. S. Dordick, Trends Biotechnol., 2008, 26, 48–54 CrossRef CAS.
- M. Matsumoto, K. Kida and K. Kondo, J. Chem. Technol. Biotechnol., 2001, 76, 1070–1073 CrossRef CAS.
- N. J. Turner, Nat. Chem. Biol., 2009, 5, 567–573 CrossRef CAS.
- M. T. Reetz, Angew. Chem., Int. Ed., 2011, 50, 138–174 CrossRef CAS.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore and K. Robins, Nature, 2012, 485, 185–194 CrossRef CAS.
- J. C. Moore and F. H. Arnold, Nat. Biotechnol., 1996, 14, 458–467 CrossRef CAS.
- M. T. Reetz, P. Soni, L. Fernandez, Y. Gumulya and J. D. Carballeira, Chem. Commun., 2010, 46, 8657–8658 RSC.
- R. J. Kazlauskas and H. K. Weber, Curr. Opin. Chem. Biol., 1998, 2, 121–126 CrossRef CAS.
- V. G. Eijsink, S. Gaseidnes, T. V. Borchert and B. van den Burg, Biomol. Eng., 2005, 22, 21–30 CrossRef CAS.
- N. Budisa, Angew. Chem., Int. Ed., 2004, 43, 6426–6463 CrossRef CAS.
- A. M. Klibanov, Nature, 2001, 409, 241–246 CrossRef CAS.
- M. G. Hoesl, C. G. Acevedo-Rocha, S. Nehring, M. Royter, C. Wolschner, B. Wiltschi, N. Budisa and G. Antranikian, ChemCatChem, 2011, 3, 213–221 CrossRef CAS.
- M. Kitagawa and Y. Tokiwa, Biotechnol. Lett., 1998, 20, 627–630 CrossRef CAS.
- Z. Jin, S. Y. Han, L. Zhang, S. P. Zheng, Y. Wang and Y. Lin, Bioresour. Technol., 2012, 130C, 102–109 Search PubMed.
- R. Gupta, N. Gupta and P. Rathi, Appl. Microbiol. Biotechnol., 2004, 64, 763–781 CrossRef CAS.
- M. Royter, M. Schmidt, C. Elend, H. Hobenreich, T. Schafer, U. T. Bornscheuer and G. Antranikian, Extremophiles, 2009, 13, 769–783 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: TTL information and 3D model, effects of other additives, chemicals and strains, protein protocols, spectrophotometric assays, general and detailed properties of TTL[1a/1b/1c] congeners. See DOI: 10.1039/c3cy20712a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |
