Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with lignin-type phenols and thermal Claisen rearrangement of linear telomers
Peter J. C.
Hausoul
ab,
Sinedu D.
Tefera
b,
Jelle
Blekxtoon
a,
Pieter C. A.
Bruijnincx
a,
Robertus J. M.
Klein Gebbink
*b and
Bert M.
Weckhuysen
*a
aInorganic Chemistry & Catalysis, Debye Institute for Nanomaterials Science, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands. E-mail: b.m.weckhuysen@uu.nl; Fax: +31 30 251 1027; Tel: +31 30 253 7400
bOrganic Chemistry & Catalysis, Debye Institute for Nanomaterials Science, Utrecht University, Universiteitsweg 99, 3584 CG, Utrecht, The Netherlands. E-mail: r.j.m.kleingebbink@uu.nl; Fax: +31 30 252 3615; Tel: +31 30 253 3120
First published on 1st November 2012
Abstract
The Pd/TOMPP-catalysed (TOMPP = tris(2-methoxyphenyl)phosphine) telomerisation of 1,3-butadiene was studied under solvent- and base-free conditions with phenolic substrates that can be potentially derived from lignin. Large differences in catalytic activity were observed, with reactivity increasing in the order of phenol, p-cresol, guaiacol, creosol and syringol. This reactivity trend can be attributed to the substrates' relative nucleophilicities, as induced by the donating effects of the p-methyl and o-methoxy substituents. The chosen reaction conditions, i.e. temperature, ligand/metal and butadiene/substrate ratios, strongly influenced both the conversion and selectivity of the reaction. Remarkably, the composition of the reaction medium, i.e. the butadiene/substrate ratio, exerted a strong influence on the linear/branched ratio. High conversions and selectivities to the linear products are obtained when excess butadiene is used. The linear telomer products could be readily converted from O-alkylated to C-alkylated phenolics via the thermal Claisen rearrangement. High conversions and selectivities were observed after 2 hours at 200 °C. Branched o-octadienyl phenols were obtained in all cases except for the syringol telomer which gave the linear p-octadienyl product exclusively.
1. Introduction
Current academic and industrial research is increasingly focused on the development of sustainable chemical processes which utilise biomass as a renewable and CO2-neutral feedstock for the production of fuels and bulk chemicals. A vast majority of these studies are concerned with the degradation and depolymerisation of the (hemi)cellulose fraction of lignocellulosic biomass to carbohydrates and other platform molecules. The subsequent conversion of these platform molecules to yield bulk chemicals or other value-added products is also increasingly being explored.1 In this regard, it has been shown that the valorisation of biomass-derived alcohols (e.g. diols, polyols, carbohydrates and starch; all derived from (hemi)cellulose) through the Pd-catalysed telomerisation of 1,3-dienes (e.g. 1,3-butadiene, isoprene and piperylene yielding telomers with two or more diene units) is a promising approach.2,3 Despite its chemical recalcitrance, also the lignin component of lignocellulosic biomass has the potential to serve as a suitable feedstock, for instance for the production of renewable phenolics.4 Depending on the specific degradation technology that is employed, a multitude of substituted aromatic compounds, which are mainly derivatives of phenol, guaiacol and syringol, may be obtained.In telomerisation, the use of phenol itself as a telogen is well known and was first reported by Smutny et al. in their pioneering studies on the Pd-catalysed telomerisation of 1,3-butadiene.5 The resulting linear telomer is a useful precursor for natural product synthesis6 and for the production of octyl phenols which are used in polymer and detergent applications. As a result, a process for the telomerisation of phenol with 1,3-butadiene has been patented several times over the years.5c,7 However, very little has been reported on the telomerisation of substituted phenols.8,9
In terms of product selectivity, it is important to note that for phenolic substrates there is also the possibility of direct C-alkylation due to the ambident nature of the substrate.8d In fact, Smutny already observed C-alkylated products during his initial investigation, but selectivities were not reported. Subsequently, the importance of this route was further demonstrated by Beller et al., who showed that optimisation of the solvent and reaction conditions allows efficient production of C-alkylated telomers for certain phenolic substrates.9
Previously, we have reported on the Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with biomass-derived substrates such as diols, sugar alcohols and carbohydrates.10 Here, we present the results of the Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with phenol (1), p-cresol (2), guaiacol (3), creosol (4) and syringol (5) (see Scheme 1) as model compounds for lignin-derived phenolic platform molecules.
 | ||
| Scheme 1 General reaction scheme for the Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with phenols (C-alkylated products not shown). | ||
2. Results and discussion
2.1 Telomerisation of phenol
To investigate the reaction conditions required for full conversion of the selected substrates, phenol (1) was selected as a model substrate. As in the previous reports on Pd/TOMPP-catalysed telomerisations, reactions were conducted neat and without the use of base additives. A metal loading of 0.02 mol% Pd(dba)2 was used with respect to the substrate and the ligand to metal ratio (P/Pd) was set at 4. The butadiene to substrate molar ratio (Bu/1) was targeted at 2 and the reaction was performed at 80, 90 and 100 °C (Table 1). At 80 °C, 70% of 1 is converted after 22 hours of reaction time, which shows that phenol reacts rather sluggishly compared to the results previously obtained for polyols.10 At 100 °C the reaction proceeds much faster, but conversion still did not exceed 70% after 4.5 hours.Increasing the butadiene to substrate ratio from 1.8 to 3.2 resulted in an increase in conversion from 70% to 99%, indicating a need for excess butadiene in the reaction mixture to reach full conversion. It is also noteworthy that the linear to branched ratios (n/iso), which range from 3.0 to 5.3, are considerably lower than those generally observed for, e.g., methanol (10 to 38).11 Conversion and selectivity were monitored in more detail by four reactions under identical conditions which were stopped after 0.5, 1, 2 and 4 hours of reaction time. The results are depicted in Fig. 1 and 2. As shown, the reaction reaches approximately 40% conversion within 0.5 hour of reaction time (TOF = 295−1 h) and an n/iso ratio of 9 is observed. Interestingly, as conversion increases to 70%, the n/iso ratio decreases to 4 during the run showing that the product composition changes over time. Pd-black formation was not observed during or after the reaction, suggesting that the homogeneous catalyst remains stable. In addition, it was also found that ethanolic solutions of the reaction mixtures, which were stored at 5 °C, slowly converted to phenol and 1,3,7-octatriene over a period of months. Finally, if isolation of the telomers from fresh reaction mixtures was attempted via high temperature vacuum distillation, this resulted partly in re-formation of phenol as well as Claisen rearrangement. All these observations show that the phenol telomer is rather unstable in the presence of the catalyst. It is also important to note that no C-alkylated products were observed in the catalytic mixtures by 1H or 13C NMR.
 | ||
| Fig. 1 Reaction profile of the Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with phenol (1) (conditions: 0.212 mol 1, Bu/1 = 1.7–1.8, 0.02 mol% Pd(dba)2, P/Pd = 4, T = 100 °C). | ||
 | ||
| Fig. 2 n/iso ratios of the Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with phenol (1) (conditions: 0.212 mol 1, Bu/1 = 1.7–1.8, 0.02 mol% Pd(dba)2, P/Pd = 4, T = 100 °C). | ||
2.2 Telomerisation of guaiacol
As with phenol, the telomerisation of guaiacol (3) was initially performed at 100 °C for 1 hour which resulted in 99% substrate conversion. To determine the intrinsic activity of 3 in more detail, the influence of the reaction temperature was studied and the obtained results are shown in Table 2. At 80 °C, the conversion reached 97% with a final n/iso ratio of 18. Conversion dropped to 75% at 60 °C, however the n/iso ratio remained roughly the same. At 40 °C, conversion was only 60%, but now also the n/iso ratio decreased to 11. These results show that guaiacol (pKa = 9.93) is a much more reactive substrate than phenol (pKa = 9.95),12 despite the fact that both substrates possess an essentially identical pKa.In contrast to phenol (Table 1), the n/iso ratios of the guaiacol telomers are considerably higher and degradation of the telomers to guaiacol and 1,3,7-octatriene was not observed for stored samples. Also, C-alkylated products were again not observed in the reaction mixtures. This shows that the linear guaiacol telomer is easily obtained in high purity. Given the increased stability of the guaiacol telomers, guaiacol was chosen as the model substrate to study the influence of reaction conditions on the product selectivity. Vollmüller et al.11 previously showed a strong influence of the ligand to metal ratio (P/Pd) on the n/iso ratio of the telomer products, in case of the Pd/PPh3-catalysed telomerisation of methanol. With our system, the P/Pd ratio was found not to influence the n/iso ratio much, as illustrated by a set of reactions run with P/Pd ratios of 1, 2, 4, and 8 (Table 3). At a P/Pd ratio of 1, substrate conversion reached 53% with an n/iso ratio of 25. At higher P/Pd ratios the conversions increased considerably, but the n/iso ratio did not change to a great extent. Therefore it appears that for the Pd/TOMPP-catalysed telomerisation of 1,3-butadiene with guaiacol the n/iso ratio is rather insensitive to the P/Pd ratio. Nonetheless, the increase in conversion at higher P/Pd ratios does show that the concentration of active species is linked to the amount of phosphine in the reaction mixture and that a more active system is obtained when excess phosphine is employed.
The influence of the butadiene to substrate ratio (Bu/3) on the outcome of the reaction was found to have a very large influence on selectivity (Table 4). Indeed, the results show that the Bu/3 ratio influences both the substrate conversion as well as the n/iso ratio. The conversion of 3 is thus directly dependent on the amount of butadiene in the system. It should be noted, however, that as in the case of phenol (see Table 1) full substrate conversion is not achieved under stoichiometric conditions. These observations are in contrast with previously published results of polyols where (near) full substrate conversions are commonly observed. The byproduct 1,3,7-octatriene was also detected, albeit always in minute amounts (below 1% wrt the total amount of butadiene).
The results at varying temperatures show that full conversion can in principle be achieved (Table 3), but it appears that product inhibition plays a role at high substrate conversions. The change in the n/iso ratio is, however, more remarkable and not as easily explained. The data show that as the Bu/3 ratio is increased from 1 to 7, the n/iso ratio considerably increases from 7 to 39. These ratios are quite high for phenolic substrates. Since the initial P/Pd ratio is equal in all cases, these results imply that the composition of the reaction medium has an influence on the equilibria between the various active species involved. Note that the reactions are run neat and that 70 wt% of the reaction medium is composed of guaiacol in the case of Bu/3 = 1, whereas if Bu/3 = 7, guaiacol comprises only 25 wt%. An extensive mechanistic study has provided further insight into the influence of the solvent composition and the species and equilibria that are responsible for the observed changes in product selectivity. These mechanistic results will be reported separately.13
2.3 Substrate screening
The other substrates (S) were comparatively screened under the general conditions of the guaiacol experiments (i.e. 0.02 mol% Pd(dba)2, P/Pd = 4, Bu/S = 4, 60 °C, 1 h). The results are listed in Table 5. As expected, the conversion of phenol (1) only amounted to 5% with an n/iso ratio of 14. The result for p-cresol (2) is fairly similar as a conversion of 8% with an n/iso ratio of 6 is observed. As reported above, guaiacol (3) is considerably more reactive and a conversion of 94% with an n/iso ratio of 22 is found. The reaction of creosol (4) gave 92% substrate conversion with an exceptionally high n/iso ratio of 52 and syringol was almost fully converted (99%) with an n/iso ratio of 39. In all cases the amount of 1,3,7-octatriene formed during the reaction remained below 1% with respect to butadiene. In contrast to the results obtained by Beller et al., C-alkylated products were not observed in any of these reactions.9These results show that only phenol (1) and p-cresol (2) are rather poor substrates in the Pd/TOMPP-catalysed telomerisation under these standard conditions. The electron-donating effect of the p-methyl substituent exerts only a small influence on the reactivity. In contrast, guaiacol (3), creosol (4) and syringol (5) are much more reactive. The increase in reactivity of these substrates must be attributed to the electron-donating effect of the o-methoxy substituents. The reactivity of the substrates clearly does not correlate with the Brønsted basicity of the substrates (see Table 5 for aqueous pKa values). Given that telomer formation proceeds via nucleophilic addition to π-allyl complexes (Scheme 2),2,3 the reactivity and hence nucleophilicity of the studied phenolates are mainly determined by the resonance donation to the conjugated p orbitals of oxygen.
![Nucleophilic addition of phenolate anions to [Pd(1–3,7,8-octa-2,7-dien-1-yl)(PR3)]+ resulting in telomer formation.](/image/article/2013/CY/c2cy20522j/c2cy20522j-s2.gif) | ||
| Scheme 2 Nucleophilic addition of phenolate anions to [Pd(1–3,7,8-octa-2,7-dien-1-yl)(PR3)]+ resulting in telomer formation. | ||
The tested phenols thus display a broad range of reactivities and selectivities in the Pd/TOMPP-catalysed telomerisation reaction. Given the rather remarkable influence of parameters such as temperature, the P/Pd ratio and the Bu/S ratio on product selectivity and yield, optimization of the reaction is easily achieved. Particularly, the high conversions and selectivities to linear products at relatively low catalyst loadings and temperatures show that this catalytic system possesses a high potential for the efficient conversion of phenolic substrates.
2.4 Claisen rearrangement
The Claisen rearrangement of allyl phenyl ethers is a well-known chemical transformation that transfers the allyl group from the oxygen to the aromatic ring.14 For telomer products this results in the formation of linear and branched octadienylphenols (Scheme 4), which are suitable starting materials for surfactant and polymer applications. The conversion of the linear phenol telomer (1a(E/Z)) via Claisen rearrangement has been described before by Muzart et al.15 Since the main product of the telomerisation reaction is the linear telomer, it was chosen to study the conversion of these pure products (i.e.1a(E/Z) to 5a(E/Z)). Given the issues discussed above regarding the stability and isolation of 1a(E/Z), Pd-free, linear telomer products were prepared via independent synthesis i.e., 1a(E/Z) was obtained from 1-iodo-2,7-octadiene16 and sodium phenolate in 69% yield with an n/iso ratio of 80 after vacuum distillation (Scheme 3). Degradation of these telomers to the corresponding phenolics was not observed. | ||
| Scheme 3 Synthesis of 1a(E/Z) from 1. Conditions: (i) tBuONa, CH3CN, RT, 1 h; (ii) 1-iodo-2,7-octadiene, CH3CN, 60 °C, overnight. | ||
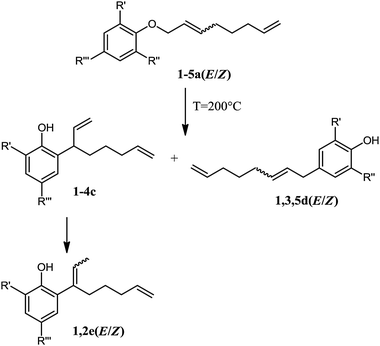 | ||
| Scheme 4 Claisen rearrangement of linear telomer products (1: R′,R′′,R′′′ = H; 2: R′,R′′ = H, R′′′ = Me; 3: R′ = OMe, R′′,R′′′ = H; 4: R′ = OMe, R′′ = H, R′′′ = Me; 5: R′,R′′ = OMe, R′′′ = H). | ||
The Claisen rearrangement of 1a–5a(EE/ZZ) was performed neat and in air at 200 °C and the progression of the reaction was followed by GC and NMR. Fig. 3 shows the results obtained for the conversion of the linear phenol telomer 1a(E/Z). After 3 hours, 97% of 1a(E/Z) is converted to three products, namely 1c, 1d(EE/ZZ) and 1e(EE/ZZ). The trans isomer (1a(EE)) is found to be most reactive as the initial trans/cis ratio (EE/ZZ) of 20 gradually decreases to 1.6 during the reaction. Initially, the reaction mainly produces the o-branched product 1c (sel. 60%) and the p-linear product 1d(EE/ZZ) (sel. 33%, E/Z = 18). After 1 hour, the ratio between 1c and 1d(EE/ZZ) remains approximately constant indicating an equilibrium between these species.
 | ||
| Fig. 3 Claisen rearrangement of the phenol linear telomer (1a(E/Z)). Conditions: neat, 200 °C (E- and Z-isomers of 1d(EE/ZZ) and 1e(EE/ZZ) are shown together for clarity). | ||
As time progresses, the relative amount of 1e(EE/ZZ) (E/Z = 11) increases to 31%. 1e(EE/ZZ) is presumably formed via an acid-catalysed isomerisation of 1c. In the case of the p-cresol telomer 2a(EE/ZZ) (Fig. 4) the reaction is slightly faster and a conversion of 99% is found after 3 hours.
 | ||
| Fig. 4 Claisen rearrangement of the p-cresol linear telomer (2a(EE/ZZ)). Conditions: neat, 200 °C (E- and Z-isomers of 2e(EE/ZZ) could not be separated). | ||
Since for 2a(EE/ZZ) the para position is blocked with a methyl group, only the o-branched products 2c and 2e(EE/ZZ) are observed. Also in this case the amount of 2e(EE/ZZ) increases during the run and a final ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between 2c and 2e(EE/ZZ) is observed at the end of the reaction. The reaction is faster with the guaiacol telomer 3a(EE/ZZ) (Fig. 5) as a conversion of 97% is observed after 2 hours. The ortho-substituted product 3c is again the main product (73%). The final selectivity to the para-substituted product 3d(EE/ZZ) amounts to 24%. The absolute amount of this product does not increase significantly after the first hour, however. In contrast to phenol and p-cresol, the isomerisation of 3c to 3e(EE/ZZ) is not observed. The creosol telomer 4a(EE/ZZ) (Fig. 6) reacts somewhat faster than 3a(EE/ZZ) resulting in 98% conversion to the o-substituted product 4c. The initial EE/ZZ ratio of 4a(EE/ZZ) of 20.4 drops to 0.3 after 2 hours. This shows again that the cis-isomer is less reactive and that the isomerisation between trans and cis is much slower than conversion via Claisen rearrangement. Finally, the syringol telomer 5a(E/Z) showed full conversion in two hours of reaction time (Fig. 7). Since both ortho positions are substituted by methoxy groups, the single product of the reaction is the p-substituted product 5d(EE/ZZ).
1 between 2c and 2e(EE/ZZ) is observed at the end of the reaction. The reaction is faster with the guaiacol telomer 3a(EE/ZZ) (Fig. 5) as a conversion of 97% is observed after 2 hours. The ortho-substituted product 3c is again the main product (73%). The final selectivity to the para-substituted product 3d(EE/ZZ) amounts to 24%. The absolute amount of this product does not increase significantly after the first hour, however. In contrast to phenol and p-cresol, the isomerisation of 3c to 3e(EE/ZZ) is not observed. The creosol telomer 4a(EE/ZZ) (Fig. 6) reacts somewhat faster than 3a(EE/ZZ) resulting in 98% conversion to the o-substituted product 4c. The initial EE/ZZ ratio of 4a(EE/ZZ) of 20.4 drops to 0.3 after 2 hours. This shows again that the cis-isomer is less reactive and that the isomerisation between trans and cis is much slower than conversion via Claisen rearrangement. Finally, the syringol telomer 5a(E/Z) showed full conversion in two hours of reaction time (Fig. 7). Since both ortho positions are substituted by methoxy groups, the single product of the reaction is the p-substituted product 5d(EE/ZZ).
 | ||
| Fig. 5 Claisen rearrangement of the guaiacol linear telomer (3a(EE/ZZ)). Conditions: neat, 200 °C. | ||
 | ||
| Fig. 6 Claisen rearrangement of the creosol linear telomer (4a(EE/ZZ)). Conditions: neat, 200 °C. | ||
 | ||
| Fig. 7 Claisen rearrangement of the syringol linear telomer (5a(E/Z)). Conditions: neat, 200 °C (E- and Z-isomers of 5a(E/Z) and 5d(EE/ZZ) are shown together due to overlap in GC, NMR data show final E/Z = 6 for 5d(EE/ZZ)). | ||
These results demonstrate that the thermal Claisen rearrangement of 2,7-octadienyl phenyl ethers proceeds efficiently at 200 °C and provides a simple and effective transformation which liberates the OH group for further functionalisation. After a single hydrogenation step, the resulting product mixtures provide suitable materials for the atom-efficient synthesis of alkyl phenol-based surfactants which are commercially produced for industrial detergent applications (Scheme 5).17 Nevertheless, the two terminal olefins of the branched products, which are typically the main products of the Claisen reaction, also offer the opportunity for use as precursors in metathesis reactions such as ring closing metathesis (RCM)18 and acyclic diene metathesis (ADMET).19 In the case of RCM the octadienyl chain can be cyclized to give a cyclohexenyl group resulting in an aryl cyclohexyl motif which forms the basis for many different pharmaceutical ingredients. With the ADMET reaction, the Claisen products may provide suitable monomers for the synthesis of sustainable phenolic rubbers. Phenolic resins are generally added as antioxidants or antidegradants to improve polymer lifetime. Hence, it is expected that the Pd-catalysed telomerisation of lignin-type phenols followed by Claisen rearrangement holds considerable potential for the sustainable production of surfactants, polymers and pharmaceuticals.
 | ||
| Scheme 5 Potential follow-up reactions for branched octadienyl phenol products obtained from Claisen rearrangement of linear telomers. | ||
3. Conclusions
The Pd/TOMPP-catalysed telomerisation of 1,3-butadiene is presented with phenolic compounds that can potentially be obtained from lignin. The results show that phenol (1) and p-cresol (2) are rather poor substrates which require elevated temperatures to reach full conversion. A very low linear to branched ratio was observed and kinetic measurements on the telomerisation of phenol show that the linear to branched ratio decreases during the run. Interestingly, C-alkylated products are not observed in these reactions. Substrates with resonance-donating groups such as guaiacol (3), creosol (4) and syringol (5) are considerably more reactive. In the case of guaiacol, variation in the ligand to metal ratio has little effect on the linear to branched ratio. However, variation in the butadiene to substrate ratio has a marked effect on the product selectivity and very high linear to branched ratios are observed when excess amounts of butadiene are used.Conversion of the linear telomer products by thermal Claisen rearrangement is found to proceed efficiently. For substrates with unsubstituted ortho positions, high selectivities towards o-branched products are observed. The formation of the para-linear product is also observed and in the case of the syringol telomer it is obtained exclusively. The rearrangement products may find use in synthetic building blocks, e.g., in the synthesis of pharmaceuticals, polymers or surfactants.
Finally, details of the catalytic studies reported here prompted further mechanistic studies focused on the influence of reaction conditions on selectivity and the equilibration of the linear to branched ratio. These studies will be reported separately.13
4. Experimental section
4.1 General remarks
All reactions were carried out under an oxygen-free, dry nitrogen atmosphere using standard Schlenk techniques unless stated otherwise. 1-Iodo-2,7-octadiene was prepared according to previously published procedures.12 Pd(dba)2 was obtained from ABCR. Phenol and p-cresol were obtained from ACROS. Guaiacol, creosol and syringol were obtained from Sigma Aldrich. 1H and 13C NMR spectra were recorded on a Varian AS400. Chemical shifts are reported in ppm relative to residual solvent signals. Calibration standards were either synthesized or isolated from catalytic mixtures. The response factor of the linear telomer was used also for the branched telomer. Response factors of Claisen products were assumed to be equal to the linear telomer. GC analysis was performed on a Shimadzu GC-2010 system with a WCOT fused silica column (25 m × 0.25 mm, CP-WAX 57CB).4.2 1H and 13C NMR numbering schemes
The numbering scheme of the linear telomers used for the assignment of the NMR spectra is depicted in Fig. 8. | ||
| Fig. 8 1H and 13C NMR numbering scheme of linear telomers 1a(E/Z) to 5a(E/Z). | ||
4.3 100 mL stainless steel autoclave telomerisation experiments
Phenol telomerisation experiments were conducted in a 100 mL stainless steel autoclave with an overhead stirrer from Parr. Substrate, catalyst and ligand were introduced into the autoclave cup and the autoclave was assembled and sealed. The autoclave was flushed two times with 10 bar N2 or argon and subsequently cooled to −70 °C using a dry ice–acetone bath. Butadiene was condensed directly in the autoclave using a calibrated flow. The total amount of butadiene added was determined gravimetrically (err. ±0.1 g). The contents were vigorously stirred and heated to the reaction temperature. After the end of the reaction, the reactor was opened to vent the remaining butadiene. The reactor was disassembled and the content was diluted with ethanol and transferred in a 100 mL volumetric flask and analysed.4.4 50 mL high pressure glass reactor telomerisation experiments
Guaiacol and substrate screening experiments were conducted in a 50 mL high pressure glass vessel with a Teflon liner and a pressure cap (15 bar) from Salm en Kip. Substrate, catalyst, ligand and a magnetic stirring bar were introduced into the glass vessel. The vessel was flushed with N2 and cooled to −20 °C using a dry ice–acetone bath. A gas inlet tube (butadiene) was placed in the vessel and the top of the vessel was closed with parafilm. Butadiene was condensed directly in the vessel using a calibrated flow and the total amount of introduced butadiene was determined gravimetrically (err. ±0.1 mg). The reactor was closed and the contents were stirred and heated to 60 °C using an oil bath and a heating plate fitted with a thermocouple. After the end of the reaction, the reactor was cooled to −20 °C and opened. The contents were diluted with toluene and transferred to a 100 mL volumetric flask and analysed.4.5 General procedure for the synthesis of 2,7-octadienyl phenyl ethers
To a stirred solution of 4.36 g (46.3 mmol) phenol in 90 mL dry acetonitrile 4.32 g (45.0 mmol) tBuONa was added and left to react for 1 hour. To this solution 9.49 g (40.4 mmol) 1-iodo-2,7-octadiene was added and left stirring overnight at 60 °C. The solution was concentrated in vacuo and the obtained residue was redissolved in Et2O and extracted with aqueous NaOH (1 M) and water. The organic phase was separated, dried over MgSO4, filtered and concentrated in vacuo. Isolation by high temperature distillation under reduced pressure gave 5.63 g (27.8 mmol, 68.8%) of the ether product.4.6 Linear telomer products
4.7 Procedure for the Claisen rearrangement
1 mL of the isolated telomer product was stirred and heated in a closed 5 mL vial to 200 °C on a heating plate fitted with an aluminium heat block and a thermocouple. 0.1 mL aliquots were taken and after dilution analysed by GC.4.8 Claisen rearrangement products
Acknowledgements
The authors thank ACTS-ASPECT for financial support.Notes and references
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- (a) A. Behr, M. Becker, T. Beckmann, L. Johnen, J. Leschinski and S. Reyer, Angew. Chem., Int. Ed., 2009, 48, 3598 CrossRef CAS; (b) S. Bouquillon, J. Muzart, C. Pinel and F. Rataboul, Top. Curr. Chem., 2010, 295, 93 CrossRef CAS.
- (a) D. Medema and R. van Helden, Recl. Trav. Chim. Pays–Bas, 1971, 90, 324 CrossRef CAS; (b) P. Grenouillet, D. Neibecker, J. Poirier and I. Tkatchenko, Angew. Chem., Int. Ed. Engl., 1982, 21, 767 CrossRef; (c) M. Camargo, P. Dani, J. Dupont, R. F. de Souza, M. Pfeffer and I. Tkatchenko, J. Mol. Catal. A: Chem., 1996, 109, 127 CrossRef CAS.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552 CrossRef CAS.
- (a) E. J. Smutny, J. Am. Chem. Soc., 1967, 89, 6793 CrossRef CAS; (b) E. J. Smutny, H. Chung, K. C. Dewhirst, W. Keim, T. M. Shryne and H. E. Thyret, Prepr. Pap. - Am. Chem. Soc., Div. Pet. Chem., 1969, 14, B100 CAS; (c) E. J. Smutny (Shell), U.S. 3518318, 1970 Search PubMed; (d) E. Smutny, Ann. N. Y. Acad. Sci., 1973, 125 CrossRef CAS.
- (a) T. Mandai, H. Yasuda, M. Kaito and J. Tsuji, Tetrahedron, 1979, 35, 309 CrossRef CAS; (b) J. Tsuji, Pure Appl. Chem., 1979, 51, 1235 CrossRef CAS; (c) J. Tsuji, T. Yamakawa and T. Mandai, Tetrahedron Lett., 1979, 39, 3741 CrossRef.
- (a) S. Hattori, H. Munakata, T. Suzuki, Y. Nishikawa and N. Imaki, (Mitsubishi) DE 1807491, 1969 Search PubMed; (b) T. M. Shyrne, (Shell) US 3530187, 1970 Search PubMed; (c) H. Chung and W. Keim, (Shell) US 3636162, 1972 Search PubMed; (d) E. Kuntz, (Rhone-Poulenc) DE 2733516, 1978 Search PubMed; (e) D. Roettger, M. Beller, R. Jackstell and H. Klein, K.-D. Wiese, (Oxeno) DE 10128144, 2002 Search PubMed; (f) E. Drent, (Shell) WO 03040065, 2003 Search PubMed.
- (a) F. J. Weigert and W. C. Drinkard, J. Org. Chem., 1973, 38, 335 CrossRef CAS; (b) J. Beger, C. Duschek, H. Füllbier and W. Gaube, J. Prakt. Chem., 1974, 316, 26 CrossRef CAS; (c) K. Kaneda, H. Kurosaki, M. Terasawa, T. Imanaka and S. Teranishi, J. Org. Chem., 1981, 46, 2356 CrossRef CAS; (d) R. Jackstell, S. Harkal, H. Jiao, A. Spannenberg, C. Brogmann, D. Röttger, F. Nierlich, M. Elliot, S. Niven, K. Cavell, O. Navarro, M. S. Viciu, S. P. Nolan and M. Beller, Chem.–Eur. J., 2004, 10, 3891 CrossRef CAS.
- A. Krotz, F. Volmüller, G. Stark and M. Beller, Chem. Commun., 2001, 195 RSC.
- (a) R. Palkovits, I. Nieddu, R. J. M. Klein Gebbink and B. M. Weckhuysen, ChemSusChem, 2008, 1, 193 CrossRef CAS; (b) R. Palkovits, I. Nieddu, C. A. Kruithof, R. J. M. Klein Gebbink and B. M. Weckhuysen, Chem.–Eur. J., 2008, 14, 8995 CrossRef CAS; (c) R. Palkovits, A. N. Parvulescu, P. J. C. Hausoul, C. A. Kruithof, R. J. M. Klein Gebbink and B. M. Weckhuysen, Green Chem., 2009, 11, 1155 RSC; (d) A. N. Parvulescu, P. J. C. Hausoul, P. C. A. Bruijnincx, R. J. M. Klein Gebbink and B. M. Weckhuysen, Catal. Today, 2010, 158, 130 CrossRef CAS; (e) P. J. C. Hausoul, P. C. A. Bruijnincx, R. J. M. Klein Gebbink and B. M. Weckhuysen, ChemSusChem, 2009, 2, 855 CrossRef CAS.
- F. Vollmüller, J. Krause, S. Klein, W. Mägerlein and M. Beller, Eur. J. Inorg. Chem., 2000, 1825 CrossRef.
- M. Ragnar, C. T. Lindgren and N.-O. Nilvebrant, J. Wood Chem. Technol., 2000, 20, 277 CrossRef CAS.
- P. J. C. Hausoul, M. Lutz, J. T. B. H. Jastzrebski, P. C. A. Bruijnincx, B. M. Weckhuysen, R. J. M. Klein Gebbink, in preparation.
- A. M. Martin Castro, Chem. Rev., 2004, 104, 2939 CrossRef.
- C. Damez, S. Bouquillon, F. Hénin and J. Muzart, Eur. J. Org. Chem., 2006, 4565 CrossRef CAS.
- P. J. C. Hausoul, A. N. Parvulescu, M. Lutz, A. L. Spek, P. C. A. Bruijnincx, R. J. M. Klein Gebbink and B. M. Weckhuysen, ChemCatChem, 2011, 3, 845 CrossRef CAS.
- U. Zoller and P. Sosis, Handbook of Detergents, Part F, CRC Press, 2009 Search PubMed.
- S. Kotha, N. G. Krishna, S. Halder and S. Misra, Org. Biomol. Chem., 2011, 9, 5597 CAS.
- (a) S. Guenther, P. Lamprecht and G. A. Luinstra, Macromol. Symp., 2010, 293, 15 CrossRef CAS; (b) H. Mutlu, A. N. Parvulescu, P. C. A. Bruijnincx, B. M. Weckhuysen and M. A. R. Meier, Macromolecules, 2012, 45, 1866 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
