Laccase—a natural source for the synthesis of benzofuro[2,3-c]pyrazolin-5-ones†
Mazaahir
Kidwai‡
*a,
Arti
Jain
a,
Abha
Sharma
b and
Ramesh Chander
Kuhad
b
aGreen Chemistry Research Laboratory, Department of Chemistry, University of Delhi, Delhi 110007, India. E-mail: kidwai.chemistry@gmail.com; Fax: +91 1127666235; Tel: +91 1127666235
bLignocellulose Biotechnology Laboratory, Department of Microbiology, University of Delhi, South Campus, New Delhi 110021, India
First published on 14th September 2012
Abstract
Enzymatic oxidation of catechols/hydroquinones in the presence of pyrazolin-5-ones as a nucleophile in aqueous solution has been investigated in detail by applying laccase as a catalyst. This is the first report for the enzymatic synthesis of a novel series of compounds. We derived some new compounds with the catechol/hydroquinone ring in moderate yields based on enzymatic synthesis in an environmentally benign aqueous solution. These types of reactions represent a milestone along the path of future sustainable green chemistry.
Introduction
Heteroaromatic compounds have attracted significant attention in the design of biologically active molecules and advanced organic materials.1,2 Hence, a practical method for the preparation of such compounds is of great interest in synthetic organic chemistry.3 Among the family of heterocyclic compounds, benzofurans are ubiquitous in nature and attractive heterocycles for new drug discovery.4,5 It is reported that benzofurans exhibit a wide range of pharmacological activities, including antitumor and antiviral activities (e.g., naturally occurring cyclopenta[b]benzofurans),6 potent, reversible and non-selective aromatase inhibitory effect (e.g., 1-[(benzofuran-2-yl)phenylmethyl]imidazoles),7 and selective cytotoxicity against tumorigenic cell lines.8 Pyrazole compounds are important systems that often exist in biologically active natural products and synthetic derivatives.9,10 3-Methyl-1-phenyl-2-pyrazoline-5-one (MCI-186) is a potent drug for many diseases.11,12 Many antitumor agents feature a pyrazolyl group as one of the main constituents. Such fused pyrazole-containing moieties, for example, indenopyrazole derivatives,13 benzofuro[3,2-c]pyrazolyl-3-amine14,15 and 3-phenylbenzofuropyrazoles,16 show high antitumor activities. These observations, together with the combination principles of drug design, make us think that incorporation of benzofurans with pharmaceutically active pyrazole may lead to super-potent pharmaceutical agents.As part of the exploration of green approaches for synthetic chemistry,17–19 the primary objective of the work in this paper, therefore, was twofold. Firstly, we applied a combination principle to design more potent molecules and secondly to explore a greener way to synthesize such types of compounds.
In recent years, the advances in greener technologies has broadened the availability of low cost enzymes. In turn, this has increased the potential application of enzymes for organic reactions. The unique properties of water in aqueous medium like high dielectric constant and cohesive energy density showed an extraordinary effect on reaction rates. Moreover, its cost-effectiveness, high abundance, non-inflammability and non-toxic nature increased its applicability.20
Copper active sites play a major role in biological and abiological dioxygen activation. Oxygen intermediates have been studied in detail for the proteins and enzymes involved in reversible O2 binding (hemocyanin), activation (tyrosinase), and four-electron reduction to water21 (multicopper oxidases).
Tyrosinase and laccase both catalyze oxidation of substrate using molecular oxygen as a terminal electron acceptor with concomitant reduction of oxygen to water. Tyrosinases oxidize their substrates by removing a pair of electrons from the substrate,22,23 whereas laccases oxidize their substrates with a single electron removal mechanism.24 Although laccases and tyrosinases show overlapping substrate specificity,25,26 the primary oxidation products in laccase-catalyzed reactions are reactive radicals, which can react further and lead to polymerization, hydration, and disproportionation.27
Due to wide substrate specificity, laccases have gained much attention over the last number of years in many industrial and environmental fields.28,29
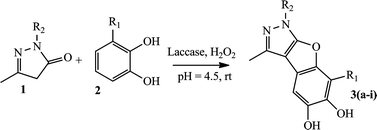
In the course of our search for efficient and green synthesis of novel benzofuropyrazoles and to explore chemo-enzymatic reactions in aqueous medium, an efficient laccase-catalyzed reaction was investigated. This reaction also required H2O2, a stoichiometric oxidant.
Results and discussion
There has been a great deal of interest in the synthesis of benzofuropyrazoles23 owing to its biological importance. A successful synthesis of benzofuro[2,3-c] pyrazoles through fusion of 1,2 or 1,4-dihydroxy benzenes with 3-methyl-1-phenyl-2-pyrazolin-5-one/3-methyl-2-pyrazolin-5-one was attempted. Here, the action of the laccase, for the in situ generation of quinones, is reported. Laccase, a multicopper oxidase, reduces oxygen (source of O2 is from the disproportionation redox reaction of H2O2 in acidic medium) to water and simultaneously performs one-electron oxidation of many aromatic substrates such as phenol and aromatic amines. Catechol/hydroquinones have more electron density at the ortho-position, so they undergo 1,4-addition reaction with pyrazolinone derivatives in the presence of laccase.The reaction conditions were optimized for the enzymatic synthesis of 3a (Scheme 1) from 1a. An experiment was carried out using 1.5 mmol of 1a and 1 mmol of 2a in 15 mL of solvent without the addition of laccase or H2O2. There was no product formation. The same was also observed when 0.5 mL of 30% of H2O2 and heat-killed laccase were added. In another experiment, when 400 U laccase were added without H2O2, only traces of product formation were observed on thin layer chromatography (TLC). The yield of 3a was significantly improved with the addition of 0.5 mL of 30% H2O2 along with 400 U of laccase. An excess of pyrazolinone was required for the reaction as quinones may undergo competing decomposition, dimerization or polymerization due to their intrinsic instability. Catechol/hydroquinones and pyrazolinone derivatives were used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio to tackle the problem.
1.5 ratio to tackle the problem.
 | ||
| Scheme 1 Model reaction between catechol and 3-methyl-1-phenyl-pyrazoline-5-one using laccase (P. cinnabarinus) in phosphate–citrate buffer of pH = 4.5 at room temperature. | ||
Table 1 shows the effect of different solvent systems on product yield. In DMSO, DMF and MeCN, no reaction was observed due to denaturation of laccase. The reaction in aqueous buffer produced higher yields when using buffer tablets of NaH2PO4, Na2HPO4, and potassium phthalate (pH 4). The lower percentage yields in other solvent systems were due to a decrease in laccase activity in immiscible organic and aqueous phases. Moreover, the domino reaction was shown to exhibit higher reactivity and selectivity in aqueous medium rather than in organic solvents. Furthermore, the effect of reaction temperature was studied by varying it from 5 °C to 60 °C. The optimal reaction temperature was found to be room temperature (25 °C). This could be attributed to the increase in the rate of decomposition and polymerization of the in situ generated quinones when a higher temperature was employed, while the insolubility of the pyrazolinone derivatives was observed at lower temperature.
| S. No | Solvent | Yieldb |
|---|---|---|
| a Reaction conditions: 1.5 mmol 1a and 1 mmol of 2a were placed in 15 mL of solvent. 400 U of laccase (P. cinnabarinus) was added and the resultant solution was stirred well for 12 h. b Isolated yield. | ||
| 1 | 1 M acetate buffer pH 4 | 20% |
| 2 | Water | — |
| 3 | THF | 22% |
| 4 | Ethanol | 20% |
| 5 | 1 M phosphate–citrate buffer pH 4.5 | 59% |
| 6 | 1 M phosphate–citrate buffer pH 7 | 48% |
| 7 | DMSO | — |
| 8 | MeCN | — |
| 9 | DMF | — |
| 10 | Methanol | 30% |
| 11 | 1,4-Dioxane | 18% |
Effect of enzyme concentration
The effect of enzyme concentration for the reaction was studied to determine the minimum amount of enzyme required for maximum formation of product. It is essential to optimize the concentration of the enzyme in the reaction mixture because it greatly influences the rate of reaction. In the present investigation it was observed that the rate of laccase catalyzed product formation increased with the increase in enzyme concentration and decreased after reaching optimal enzyme concentration. A similar trend was observed in all the derivatives. The optimal enzyme concentration was found to be 400 U and above, where there was no appreciable change in yield of the product (Table 2). Hence, 400 U of laccase is used as a standard concentration for further reactions.| S. No | Concentration of enzyme | Yieldb |
|---|---|---|
| a Reaction conditions: 1.5 mmol 1a and 1 mmol of 2a were placed in 15 mL of buffer solution. Different units of laccase were added and the resultant solution was stirred well for 12 h. b Isolated yield. | ||
| 1 | 100 U | 21% |
| 2 | 200 U | 30% |
| 3 | 400 U | 59% |
| 4 | 500 U | 61% |
After developing the optimum reaction conditions, the reactions of a variety of catechol/hydroquinoine were examined (Schemes 2 and 3) and these results are explained comprehensively (Table 3).
 | ||
| Scheme 2 | ||
 | ||
| Scheme 3 | ||
| S. No. | Entry no. | R1 | R2 | Yieldb [%]3a–i | Yieldb [%]5a–i | Time (h) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1.5 mmol 1 and 1 mmol of 2 or 4 were placed in 15 mL of solvents. 400 U of laccase was added and the resultant solution was stirred well for 12 h. b Yield refers to the isolated yield. | ||||||
| 1 | A | H | C6H5 | 59 | 61 | 12 |
| 2 | B | –CH3 | C6H5 | 55 | 56 | 12 |
| 3 | C | –OCH3 | C6H5 | 42 | 45 | 12 |
| 4 | D | –C(CH3)3 | C6H5 | — | — | 12 |
| 5 | E | F | C6H5 | — | — | 12 |
| 6 | F | H | H | 53 | 56 | 12 |
| 7 | G | –CH3 | H | 50 | 50 | 12 |
| 8 | H | –OCH3 | H | 40 | 41 | 12 |
| 9 | I | –C(CH3)3 | H | — | — | 12 |
The rate of reaction and nature of product is dependent on the nature and position of the substituted group on the catechol ring. The presence of bulky groups such as tert-butyl on the catechol ring causes a decrease in rate of reaction. Furthermore 3-flouro-1,2-benzoquinone, which has a strong electron withdrawing group (F), did not provide any desired product. This reactivity pattern may be caused by side reactions of highly reactive quinone.
Besides 3-substituted catechols, 4-substituted catechol such as 4-methyl/4-chlorocatechol or 3,4-dihydroxybenzoic acid were also used for the reaction, but the presence of substituted groups in the C-4 position of the catechol ring, which is a reactive site of o-benzoquinones, causes a decrease in the rate of reaction and stops the reaction at a particular stage and does not lead to the final desired product.
Experimental section
3-Methyl-1-phenyl-pyrazolin-5-one, 3-methyl-1H-pyrazolin-5-one, various catechols and hydroquinones were purchased from Aldrich. Guaiacol was purchased from Hi Media Laboratories Pvt. Ltd. (Mumbai, India). Q-Sepharose was purchased from Sigma (St. Louis, USA). All media components and chemicals used were of analytical grade. All reactions and purity of dyes were monitored by thin layer chromatography (TLC) using aluminium plates coated with silica gel (Merck) employing ethylacetate and hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). The isolated products were further purified by column chromatography using aluminium oxide (Sigma–Aldrich 24, 217-9, 70, 35-70, mesh 40 Ao surface area 675 m2 g−1). IR spectra were recorded on a Perkin–Elmer FTIR-1710 spectrophotometer using Nujol film and KBr pellet. 1H NMR were recorded on a JEOL JNMECX 400 P FT NMR system using TMS as an internal standard. The chemical shift values are recorded on the δ scale.
7). The isolated products were further purified by column chromatography using aluminium oxide (Sigma–Aldrich 24, 217-9, 70, 35-70, mesh 40 Ao surface area 675 m2 g−1). IR spectra were recorded on a Perkin–Elmer FTIR-1710 spectrophotometer using Nujol film and KBr pellet. 1H NMR were recorded on a JEOL JNMECX 400 P FT NMR system using TMS as an internal standard. The chemical shift values are recorded on the δ scale.
Microorganisms
The laccase produced from the basidiomycetous fungus, Pycnoporous cinnabarinus, was obtained from the Lignocellulose Biotechnology Laboratory, University of Delhi, South Campus, New Delhi. The fungal cultures were grown and maintained on malt extract agar (MEA)30 containing (g L−1): Malt extract 20.0; KH2PO4 0.5; MgSO4·7H2O, Ca(NO3)2·4H2O 0.5; agar 20.0 (pH 5.4) at 30 °C. Pure cultures were stored at 4 °C and subcultured every fortnight.Isolation of laccase
Laccase production was carried out using Pycnoporous cinnabarinus under solid-state fermentation conditions. 1000 g of air dried wheat bran was spread in separately sterilized enamel trays (78 × 51 × 8.1 cm3) to about 1.0 cm thick and was moistened with mineral salt solution containing (g L−1): KH2PO4 0.5; MgSO4·7H2O, Ca(NO3)2·4H2O 0.5; agar 20.0 (pH 5.4) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of solid substrate to moisture. The trays were put in autoclavable poly bags and sterilized at 121 °C (15 psi) for 30 minutes. The substrate was inoculated with 0.4% w/w of fungal pellets of P. cinnabarinus in separate trays, respectively. The trays were incubated for 168 hours at 30 ± 1 °C in a 70% relative humidity chamber. The laccase from the above three organisms was harvested by adding 0.1 mol L−1 citrate phosphate buffer (pH 5.4) in 1
2 ratio of solid substrate to moisture. The trays were put in autoclavable poly bags and sterilized at 121 °C (15 psi) for 30 minutes. The substrate was inoculated with 0.4% w/w of fungal pellets of P. cinnabarinus in separate trays, respectively. The trays were incubated for 168 hours at 30 ± 1 °C in a 70% relative humidity chamber. The laccase from the above three organisms was harvested by adding 0.1 mol L−1 citrate phosphate buffer (pH 5.4) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of solid substrate to buffer. The mixture was homogenized for 30 minutes at 30 °C, 200 rpm. The solid biomass residues were separated from the suspensions by filtration through muslin cloth and the filtrate obtained was centrifuged at 10
5 ratio of solid substrate to buffer. The mixture was homogenized for 30 minutes at 30 °C, 200 rpm. The solid biomass residues were separated from the suspensions by filtration through muslin cloth and the filtrate obtained was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 20 minutes at 4 °C. The supernatants obtained were used as the source of crude enzyme preparations.
000 × g for 20 minutes at 4 °C. The supernatants obtained were used as the source of crude enzyme preparations.
Purification of laccase
The crude laccase enzyme extracts (1000 mL) from P. cinnabarinus was precipitated by adding ammonium sulphate (40–80% cut off) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 20 minutes at 4 °C. The precipitates were resuspended in 50 mM citrate phosphate buffer (pH 5.4) and dialyzed against the same buffer. These partially purified enzyme samples, 100 mL from each fungus, were loaded on an anion exchange Q-Sepharose column (Sigma Aldrich, St. Louis, MO, USA) separately and equilibrated with 10 mM Tris-HCl buffer (pH 7.5). The proteins were eluted by NaCl gradient (0–0.5 M dissolved in equilibrating buffer) at a flow rate of 1 mL min−1 with each of the 1 mL fractions. Fractions with laccase activity were pooled and concentrated using 10 kDa filter membrane (Vivaspin, Vivascience, Sartorius Group, Stone house, UK) at 4 °C and assayed for laccase activity.
000 × g for 20 minutes at 4 °C. The precipitates were resuspended in 50 mM citrate phosphate buffer (pH 5.4) and dialyzed against the same buffer. These partially purified enzyme samples, 100 mL from each fungus, were loaded on an anion exchange Q-Sepharose column (Sigma Aldrich, St. Louis, MO, USA) separately and equilibrated with 10 mM Tris-HCl buffer (pH 7.5). The proteins were eluted by NaCl gradient (0–0.5 M dissolved in equilibrating buffer) at a flow rate of 1 mL min−1 with each of the 1 mL fractions. Fractions with laccase activity were pooled and concentrated using 10 kDa filter membrane (Vivaspin, Vivascience, Sartorius Group, Stone house, UK) at 4 °C and assayed for laccase activity.
Enzyme assay
Guaiacol was used as a substrate for assaying laccase activity. One unit of laccase was defined as the change in absorbance of 0.01 ml−1 min−1 at 470 nm.31,32General procedure for the reaction of pyrazoline-5-ones and catechols/hydroquinones
Catechol or hydroquinone (1 mmol) and pyrazolinone (1.5 mmol) were placed in 15 mL of buffer solution. Then, purified laccase (400 U) was added to the resultant solution and stirred well. The progress of the reaction was monitored by TLC examination at an interval of every 30 min. Upon completion of the reaction, the reaction mixture was extracted with ethylacetate (3 × 15). The organic layer was dried over anhydrous Na2SO4. The product was purified by column chromatography using aluminium oxide as a stationary phase and hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (80
ethyl acetate (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as a mobile phase. Products were unambiguously characterized by spectral data.
20) as a mobile phase. Products were unambiguously characterized by spectral data.
Characterization of the products
Conclusion
The scope of laccase catalysed reaction for the coupling of catechols/hydroquinones and 3-methyl-1-phenyl-pyrazolin-5-one/3-methyl-pyrazolin-5-one has been investigated. This reaction leads to a new series of benzofuropyrazole derivatives which is fruitful for the further exploration of these derivatives. This methodology is tunable for catalyst and solvent to integrate the reaction and facilitate the reduction of waste, with usage of more benign solvents. Hence, in addition to the simple reaction conditions, easy work up procedure makes our methodology a valid contribution to the existing processes in the field of enzymatic catalysis.Acknowledgements
The authors (M. Kidwai and A. Jain) are thankful to UGC New Delhi for providing financial assistance. The authors (A. Sharma and R.C. Kuhad) acknowledge financial support from DBT.Notes and references
- M. S. M. Ahmed, K. Kobayashi and A. Mori, Org. Lett., 2005, 7, 4487–4489 CrossRef CAS.
- M. Kidwai, R. Poddar, S. Diwaniyan and R. C. Kuhad, Adv. Synth. Catal., 2009, 351, 589–595 CrossRef CAS.
- G. R. Green, J. M. Evans and A. K. Vong, in Comprehensive Heterocyclic Chemistry 11, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon press, Oxford, 1995, vol. 5, p. 469 Search PubMed.
- M. Ono, H. Kawashima, A. Nonaka, T. Kawai, M. Haratake, H. Mori, M.-P. Kung, H. F. Kung, H. Saji and M. Nakayama, J. Med. Chem., 2006, 49, 2725–2730 CrossRef CAS.
- S. M. S. Atta, D. S. Farrag, A. M. K. Sweed and A. H. Abdel-Rahman, Eur. J. Med. Chem., 2010, 45, 4920–4927 CrossRef CAS.
- S. Kim, A. A. Salim, S. M. Swanson and A. D. Kinghorn, Anti-Cancer Agents Med. Chem., 2006, 6, 319–345 CrossRef CAS.
- R. Whomsley, E. Fernandez, P. J. Nicholls, H. J. Smith, P. Lombardi and V. Pestellini, J. Steroid Biochem. Mol. Biol., 1993, 44, 675–676 CrossRef CAS.
- I. Hayakawa, R. Shioya, T. Agatsuma, H. Furukawa, S. Naruto and Y. Sugano, Bioorg. Med. Chem. Lett., 2004, 14, 455–458 CrossRef CAS.
- T. L. Glichrist, Heterocyclic Chemistry, Addison Wesely Longman, England, 3rd edn, 1998 Search PubMed; D. Lednicer, Strategies for organic drugs synthesis and design, Wiley and Sons, New York, 1998, ch. 8 Search PubMed.
- M. Kidwai, A. Jain and R. Poddar, J. Organomet. Chem., 2011, 696, 1939–1944 CrossRef CAS.
- H. Kawai, H. Nakai, M. Suga, S. Yuki, T. Watanabe and Ken-Itchi saito, J. Pharmacol. Exp. Ther., 1997, 281, 921–927 CAS.
- S. Murota, I. Morita and N. Suda, Ann. N. Y. Acad. Sci., 1990, 598, 182–187 CrossRef CAS.
- E. W. Yue, C. A. Higley, S. V. DiMeo, D. J. Carini, D. A. Nugiel, C. Benware, P. A. Benfield, C. R. Burton, S. Cox, R. H. Grafstrom, D. M. Sharp, L. M. Sisk, J. F. Boylan, J. K. Muckelbauer, A. M. Smallwood, H. Y. Chen, H. C. Chang, S. P. Seitz and G. L. Trainor, J. Med. Chem., 2002, 45, 5233–5248 CrossRef CAS.
- J. Talley and R. Stephen, WO Patent, 96/09304, 1996 [Chem. Abstr., 125, 114605–114609] Search PubMed.
- F. S. Caruso, WO Patent, 00/29023, 2000 [Chem. Abstr., 133, 9109–9113] Search PubMed.
- K. Murata, H. Kumagai, T. Kawashima, K. Tamitsu, M. Irie, H. Nakajima, S. Suzu, M. Shibuya, S. Kamihira, T. Nosaka, S. Asano and T. Kitamura, J. Biol. Chem., 2003, 278, 32892–32898 CrossRef CAS.
- M. Kidwai, S. Bhardwaj, N. K. Mishra, A. Jain, A. Kumar and S. Mozzumdar, Catal. Sci. Technol., 2011, 1, 426–430 CAS.
- M. Kidwai, A. Jain and S. Bhardwaj, Mol. Diversity, 2012, 16, 121–128 CrossRef CAS.
- M. Kidwai, A. Jain, A. Sharma and R. C. Kuhad, Water Sci. Technol., 2012, 62(2), 385–393 Search PubMed.
- M. Kidwai and A. Jain, Appl. Organomet. Chem., 2011, 25, 620 CrossRef CAS.
- E. I. Solomon, P. Chen, M. Metz, S.-K. Lee and A. E. Palmer, Angew. Chem., Int. Ed., 2001, 40, 4570–4590 CrossRef CAS.
- K. Lerch, Mol. Cell. Biochem., 1983, 52(2), 125–138 CrossRef CAS.
- D. A. Robb Tyrosinase, in Copper Proteins and Copper Enzymes, ed. R. Lontie, CRC Press, Inc., Boca Raton, FL, 1984, vol. 2, pp. 207–270 Search PubMed.
- I. Bento, M. Arménia Carrondo and P. F. Lindley, JBIC, J. Biol. Inorg. Chem., 2006, 11, 539–547 CrossRef CAS.
- A. Käärik, Bibl Mycol, 1965, 81, 1–151 Search PubMed.
- R. M. Dawley and W. H. Flurkey, Phytochemistry, 1993, 33, 281–284 CrossRef CAS.
- C. Thurston, Microbiology, 1994, 40, 19–26 CrossRef.
- S. Witayakran and A. J. Ragauskas, Adv. Synth. Catal., 2009, 351, 1187 CrossRef CAS.
- M. Kidwai, A. Jain, A. Sharma and R. C. Kuhad, J. Mol. Catal. B: Enzym., 2012, 74, 236–240 CrossRef CAS.
- Z.-Z. Zhou, M. Zou, C.-Q. Zhou, Y.-H. Deng, M.-H. Chen, C.-P. Gu, Z.-H. Jiang, W.-H. Chen and S.-W. Liu, Chem. Pharm. Bull., 2011, 59(8), 1057–1061 CrossRef CAS.
- K. Vasdev and R. C. Kuhad, Folia Microbiol., 1994, 39, 61–64 CrossRef CAS.
- S. Dhawan and R. C. Kuhad, Bioresour. Technol., 2002, 84(1), 35 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and compound characterisation. See DOI: 10.1039/c2cy20452e |
| ‡ Presently working as Vice-chancellor, Jiwaji University, Gwalior (M. P.), India (on deputation from Department of Chemistry, University of Delhi, Delhi-110007, India). |
| This journal is © The Royal Society of Chemistry 2013 |
