Crystal engineering of topochemical solid state reactions
Abstract
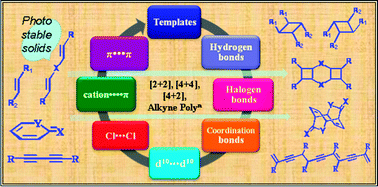
Solid state reactions offer a unique opportunity for synthesizing complex molecules with amazing regio- and stereo-specificity otherwise difficult to synthesize by conventional organic synthetic methodologies. In particular, the solid state [2 + 2] reaction is a well studied reaction in terms of a crystal engineering perspective. The main challenge in performing solid state reactions is bringing the molecules into reactive orientations such that the reactive groups are within a certain proximal distance. The out surge in organic and inorganic crystal engineering studies offers several strategies for bringing the molecules together in the crystal lattice. These strategies include from weak interactions (halogen bonds, halogen⋯halogen, π⋯π, cation⋯π) to strong hydrogen bonds (O–H⋯O, N–H⋯O, O–H⋯N and N–H⋯N) to coordination complexes/polymers. To date many studies are available which use such strategies for conducting single, double, triple or multiple [2 + 2] reactions, [4 + 4] reactions, Diels–Alder reactions and polymerization reactions of acetylene molecules. Some of the crystal engineering strategies include the use of external templates which can be removed after the reaction and some of the other strategies deals with the modification of reactant molecules. In this review, the current status of various strategies will be outlined with respect to the nature of the interactions involved.

 Please wait while we load your content...
Please wait while we load your content...