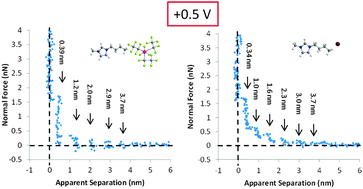
Colloid probe atomic force microscopy (AFM) force measurements are used to elucidate the effect of variation in the cation alkyl chain length and the anion species on IL nanostructure at Au(111) surfaces as a function of potential. Four ionic liquids (ILs) are examined: 1-ethyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ([EMIM] FAP), 1-butyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ([BMIM] FAP), 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ([HMIM] FAP) and 1-butyl-3-methylimidazolium iodide ([BMIM] I). The step-wise force–distance profiles show the ILs adopt a multilayered morphology, with stronger near surface structure present at more biased potentials. The results suggest that the innermost (interfacial) layer is enriched in counter ions strongly bound to the Au(111) surface. For ILs with FAP− anions, the cations in the interfacial layer at negative potentials pack more neatly than the anions at positive potentials, and thus more effectively template structure in subsequent layers. [BMIM] FAP has the weakest interfacial structure. [EMIM] FAP has stronger interfacial structure because the imidazolium rings of [EMIM]+ cations in the interfacial layer are orientated towards the Au(111) surface, and this more parallel orientation is favourable for templating structure. [HMIM] FAP is more strongly structured than [BMIM] FAP because the longer cation alkyl chain increases solvophobic interactions which lead to better defined near surface structure. The response of [BMIM] I to changes in potential is opposite to that of the FAP− ILs. [BMIM] I interfacial nanostructure is stronger at positive potentials, because I− anions pack more neatly at the Au(111) surface than [BMIM]+ cations, which templates stronger structure in subsequent layers.