Using high resolution electronic spectroscopy to probe the effects of ring twist on charge transfer in 2-phenylindole and N-phenylcarbazole†
Abstract
High resolution
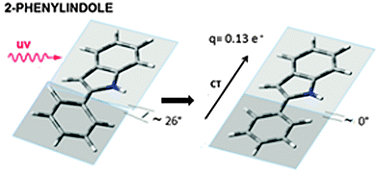
- This article is part of the themed collection: Spectroscopy and dynamics of medium-sized molecules and clusters

 Please wait while we load your content...
Please wait while we load your content...