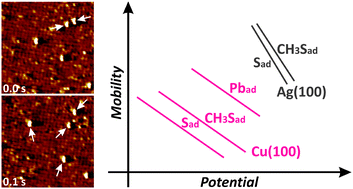
The surface diffusion of adsorbates at electrochemical interfaces is studied by in situ scanning tunneling microscopy with high temporal resolution, using sulfur and methyl thiolate on c(2 × 2) Cl covered Cu(100), Ag(100), and Au(100) electrode surfaces in 0.01 M HCl solution as an example. While on Au(100) quantitative studies were not possible because of the slow dynamics and high surface defect density, on Cu(100) and Ag(100) a pronounced exponential increase of the jump rates of isolated adsorbates toward more negative potentials was found, indicating a linear decrease of the tracer diffusion barriers with potential. The potential dependence is independent of the adsorbate species, but differs for Cu(100) and Ag(100) substrates. These trends can be explained by electrostatic contributions to the diffusion barrier, caused by the interaction of the adsorbates with the field of the electrochemical double layer, if the presence of the chloride coadsorbate layer is taken into account.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...