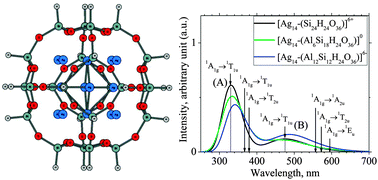
Optical properties of silver Agn nanoclusters are demonstrated to be dependent on their size, structure and charge state. It is found that when being contained in the sodalite cavity of LTA zeolite the tetradecanuclear hexacation silver cluster Ag146+ is stable. Its lower-lying states and optical spectrum are theoretically determined using the quantum chemical TD-DFT method. Its ground state possesses an outer-shell electron configuration of A1g2T2g6 mimicking the s2p6 valence of noble gas atoms. These frontier orbitals are constructed from 5s,5p(Ag)-AOs with contributions from framework oxygen atoms. Light absorption of Ag146+ embedded in the sodalite cage which is characterized by strong peaks centered at 331 and 476 nm (transitions 5s,p(Ag) → 5s,p(Ag)) leads to much longer wavelength emission. The sodalite cage, as a container, stabilizes the central Ag146+ cluster by electrostatic attraction. The absorption spectrum of the isovalent neutral Ag8 cluster embedded inside the same sodalite cavity is also simulated using TD-DFT and CASPT2 methods. This absorption spectrum which is similar to that of the Ag146+ cluster has two absorption bands in the near UV and visible regions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...