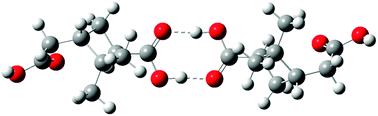
Dimers and higher order oligomers, whether in the gas or particle phase, can affect important atmospheric processes such as new particle formation, and gas-particle partitioning. In this study, the thermodynamics of dimer formation from various oxidation products of α-pinene ozonolysis are investigated using a combination of Monte Carlo configuration sampling, semi-empirical and density functional theory (DFT) quantum mechanics, and continuum solvent modeling. Favorable dimer formation pathways are found to exist in both gas and condensed phases. The free energies of dimer formation are used to calculate equilibrium constants and expected dimer concentrations under a variety of conditions. In the gas phase, favorable pathways studied include formation of non-covalent dimers of terpenylic acid and/or cis-pinic acid and a covalently-bound peroxyhemiacetal. Under atmospherically relevant conditions, only terpenylic acid forms a dimer in sufficient quantities to contribute to new particle formation. Under conditions typically used in laboratory experiments, several dimer formation pathways may contribute to particle formation. In the condensed phase, non-covalent dimers of terpenylic acid and/or cis-pinic acid and covalently-bound dimers representing a peroxyhemiacetal and a hydrated aldol are favorably formed. Dimer formation is both solution and temperature dependent. A water-like solution appears to promote dimer formation over methanol- or acetonitrile-like solutions. Heating from 298 K to 373 K causes extensive decomposition back to monomers. Dimers that are not favorably formed in either the gas or condensed phase include hemi-acetal, ester, anhydride, and the di(α-hydroxy) ether.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...