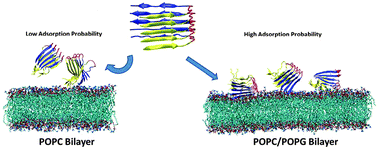
Interaction of p3 (Aβ17–42) peptides with cell membranes is crucial for the understanding of amyloid toxicity associated with Alzheimer's disease (AD). Such p3–membrane interactions are considered to induce the disruption of membrane permeability and integrity, but the exact mechanisms of how p3 aggregates, particularly small p3 oligomers, induce receptor-independent membrane disruption are not yet completely understood. Here, we investigate the adsorption, orientation, and surface interaction of the p3 pentamer with lipid bilayers composed of both pure zwitterionic POPC (palmitoyl-oleoyl-phosphatidylcholine) and mixed anionic POPC–POPG (palmitoyl-oleoyl-phosphatidylglycerol) (3 : 1) lipids using explicit-solvent molecular dynamics (MD) simulations. MD simulation results show that the p3 pentamer has much stronger interactions with mixed POPC–POPG lipids than pure POPC lipids, consistent with experimental observation that Aβ adsorption and fibrillation are enhanced on anionic lipid bilayers. Although electrostatic interactions are main attractive forces to drive the p3 pentamer to adsorb on the bilayer surface, the adsorption of the p3 pentamer on the lipid bilayer with C-terminal β-strands facing toward the bilayer surface is a net outcome of different competitions between p3 peptides–lipid bilayer and ions–p3–bilayer interactions. More importantly, Ca2+ ions are found to form ionic bridges to associate negatively charged residues of p3 with anionic headgroups of the lipid bilayer, resulting in Aβ–Ca2+–PO4− complexes. Intensive Ca2+ bound to the lipid bilayer and Ca2+ ionic bridges may lead to Ca2+ hemostasis responsible for neuronal dysfunction and death. This work provides insights into the mutual structure, dynamics, and interactions of both Aβ peptides and lipid bilayers at the atomic level, which expand our understanding of the complex behavior of amyloid-induced membrane disruption.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...