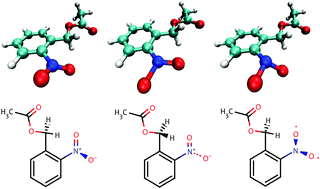
ortho-Nitrobenzylacetate (oNBA) is one of the smallest caged-compounds utilizing the famous ortho-nitrobenzyl caging group and thus serves here as a proto-typical example. Crucial for efficient uncaging of, here, acetate from oNBA is the formation of the aci-form of the nitro-group, which can be formed via excited-state intramolecular hydrogen transfer (ESIHT) in the lowest excited singlet S1 or the triplet T1 state. Using state-of-the-art quantum chemical methods, the efficiency of singlet and triplet ESIHT in aci-formation is investigated and the results are discussed in comparison to previous calculations on related caged-compounds as well as with respect to recent experimental findings. It is shown that the majority of the excited molecules undergo singlet ESIHT, which is generally inefficient with respect to aci-formation and subsequent uncaging, while only a minority of excited molecules undergo intersystem crossing and triplet ESIHT, which eventually leads to kinetically stable aci-isomers of oNBA resulting in final uncaging. Our computational results explain all existing experimental findings on ortho-nitrobenzylic caging groups conclusively and general synthetic strategies for their improvement are suggested.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...