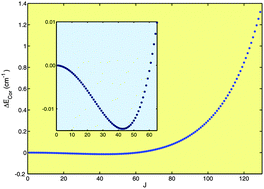
Explicitly correlated coupled cluster theory at the CCSD(T*)-F12b level (T. B. Adler, G. Knizia, and H.-J. Werner, J. Chem. Phys., 2007, 127, 221106) and two precise spectroscopic parameters (K. Kawaguchi, J. Chem. Phys., 1988, 88, 4186) were used to construct an accurate near-equilibrium analytical potential energy function (PEF) for the highly anharmonic centrosymmetric hydrogen-bonded complex ClHCl− (Re = 3.1153 Å). From variational calculations with that PEF, a large number of rovibrational energies of different isotopologues up to high values of the rotational quantum number J was obtained. Theory helped with the assignment of lines observed by