 Open Access Article
Open Access ArticleSpontaneous racemic resolution – towards control of molecular recognition nature†
Agata
Białońska
* and
Zbigniew
Ciunik
Faculty of Chemistry, University of Wrocław, 14. F. Curie-Joliot, Wrocław, Poland. E-mail: agata.bialonska@chem.uni.wroc.pl; Fax: +48 71 328 2348; Tel: +48 71 375 7388
First published on 17th June 2013
Abstract
Based on the nature of the intermolecular interactions in the crystals of racemic and enantiomeric N-(3,5-dinitrobenzoyl)asparagine, spontaneous racemic resolution was achieved by changing the ability of the compound for supramolecular synthon propagation under the control of the solvent dielectric constant.
It seems to be a paradox of life that the most important biomolecules, like proteins or DNA, are built up of homochiral units, while only a small number of racemic compounds undergo spontaneous separation resulting from conglomerate crystallization. Spontaneous separation occurs more frequently in 2D solids than in 3D crystals.1 Favorable crystallization of racemates has been explained by various factors, like a dense packing efficiency or the greater stability of a racemate (compared to a conglomerate).2 However, following Brock et al., the difference in the behavior of racemic/chiral pairs in a group of resolvable enantiomers can be attributed to statistical bias resulting from the fact that this group contains pairs in which the racemic crystal is markedly more stable than the chiral one, but no pairs in which the racemic crystal is markedly less stable.3 They showed that a kinetic factor can be important during crystallization from a racemic solution or melt. One enantiomer might inhibit the formation of the nuclei of the (chiral) crystals of the opposite enantiomer and acts as a tailor-made impurity on the subsequent growth phase of the chiral crystals. It is usually accepted that about 90% of compounds that can crystallize in either racemic or chiral space groups prefer the former.2 Nevertheless, there are known cases in which spontaneous separation occurs without any special treatment.4 One of the most common cases is ammonium sodium tartrate tetrahydrate.5 Asparagine is also one of the compounds which undergoes spontaneous separation.6 It was shown that the symmetry breaking of DL-asparagine induces enantioselective crystallization of other amino acids.7 The use of both chiral additives and crystal interfaces for spontaneous resolution are well documented.8
An important consequence of the homochirality of living systems is the ability to distinguish opposite enantiomers of a given compound during molecular recognition leading, for example, to various smell and taste sensations as well as various pharmacological effects.9 According to the great cognitive and industrial importance of chiral components, various techniques which allow us to obtain enantiopure compounds have been developed. Some of them use asymmetric synthesis, while a significant majority are based on racemic mixture separations.2 Among the latter, fractional crystallization of diastereomeric salts belongs to the most popular and most frequently applied techniques. Similarly, like it is observed in a living system, this technique is based on the ability of enantiopure (resolving agent) compounds to distinguish opposite enantiomers during molecular recognition, which is manifested in the different physical properties of the resulting diastereomeric salts.
The knowledge of molecular recognition during racemic resolution can be supportive in the design of conditions for a given and other racemic resolutions, which are still performed by trial and error. For this reason, we have examined the mechanisms of molecular recognition during racemic resolution accomplished by the fractional crystallization of diastereomeric salts of two stereochemically related resolving agents (brucine and strychnine) with various amino acid derivatives. In most cases, the stereorelated resolving agents form common brucinium or strychninium corrugated layers.10 In most cases, brucine or strychnine play the role of a host. However, anions linked to each other by a set of hydrogen bonds defined by a supramolecular synthon can reverse the host–guest functionalities of the resolving agent and resolved compound.11 Propagation of a supramolecular synthon that leads to the heterochiral self-assembly of a resolved compound can take effect in a double salt formation.12 In some cases, the synthon propagation leads to the homochiral self-assembly of a resolved compound and the resulting self-assembly plays the role of a host in the molecular recognition. This implies that the racemic resolution depends upon the propagation of the supramolecular synthon between the molecules of the resolved compound and furthermore, on the chirality of a resolving agent.
In the racemic resolution of N-(4-nitrobenzoyl)asparagine by applying brucine, the N-(4-nitrobenzoyl)-L-asparaginate anions play the role of hosts in the recognition, being self-assembled into helical ribbons.11 The helical ribbons are stabilized by a set of hydrogen bonds defined by the supramolecular synthon SS-N1 (see the scheme in Fig. 1). The racemic resolution of N-(3,5-dinitrobenzoyl)asparagine13 using brucine or strychnine as a resolving agent was performed in a similar way: 100 mg of the resolving agent and an equimolar amount of the asparagine derivative were dissolved in 10 mL of solvent (ethanol or methanol) and the samples were left to crystallize by solvent evaporation at room temperature. During the racemic resolution of N-(3,5-dinitrobenzoyl)asparagine by applying strychnine, the propagation of the same supramolecular synthon (SS-N1) is observed for the N-(3,5-dinitrobenzoyl)-L-asparaginate anions in the later fractions (Fig. 1a, S3 and S4 and Table S1 in the ESI†).14–17 When brucine is applied, the N-(3,5-dinitrobenzoyl)-D-asparaginate anions which are observed in the first crystalline fraction of the suitable racemic resolution (see Table S1 in the ESI†) are linked to each other by a set of hydrogen bonds, mediated, again, by the same (SS-N1) supramolecular synthon (see Fig. 1b, S3 and S4 in the ESI†).14–16,18
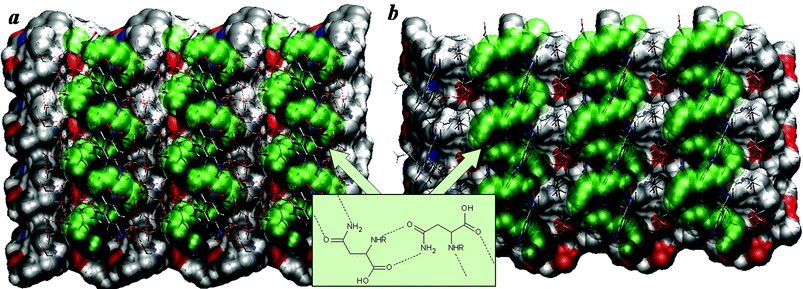 | ||
| Fig. 1 Helical chains of the hydrogen bonded N-(3,5-dinitrobenzoyl)asparaginate anions mediated by the SS-N1 supramolecular synthon (scheme) and their orientation to a surface of a) the strychninium and b) brucinium self-assemblies in a) strychninium N-(3,5-dinitrobenzoyl)-L-asparaginate pentahydrate and b) brucinium N-(3,5-dinitrobenzoyl)-D-asparaginate ethanol solvate hydrate (O – red, N – blue, C – silver, H – white; the side chain of the asparagine derivative is marked by green).19 | ||
Statistical analysis reveals that the supramolecular synthon SS-N1 is not particularly favored for compounds containing the asparagine fragment. Have the brucine or strychnine moieties induced the supramolecular synthon propagation or is the supramolecular synthon characteristic of the few following asparagine derivatives: N-(4-nitrobenzoyl),11 (S)-N2-((4-methoxyphenyl)sulfonyl),20 (S)-N-(4-tolylsulfonyl)21 and N-(3,5-dinitrobenzoyl)asparagine derivatives (Table S1 in the ESI†) regardless of the presence of a chiral agent? The presence of the chiral agent could preliminarily induce the separation of the racemic asparagine derivative and the formation of the self-assemblies defined by a supramolecular synthon. It is also likely that the self-assembled asparagine derivative formation is unrelated to the presence of the chiral agent.
To find out what role the resolving agent plays (brucine or strychnine) in the formation of the anionic self-assemblies defined by a supramolecular synthon, crystallizations from aqueous solutions containing racemic or optically pure N-(3,5-dinitrobenzoyl)asparagine were performed at room temperature by the slow evaporation of the solvent. In the experiment in which the racemic asparagine derivative was used, crystals belonging to the monoclinic space group P21/c precipitated (DNBN-rac)‡14–16 while crystals belonging to the orthorhombic space group P212121 precipitated when using the enantiomeric asparagine derivative (DNBN-e).‡14–16
DNBN-e reveals that the asparagine derivative molecules are linked to each other by a set of hydrogen bonds mediated by the supramolecular synthon SS-N1, resulting in the helical self-assembly formation (see Fig. 2a and Table S2 in the ESI†). Consecutive helical ribbons are connected by other hydrogen bonds, resulting in a bilayer structure formation. The 3,5-dinitrobenzoyl group is directed outward of the bilayer and is engaged in N⋯O interactions with the 3,5-dinitrobenzoyl group of neighboring bilayers.
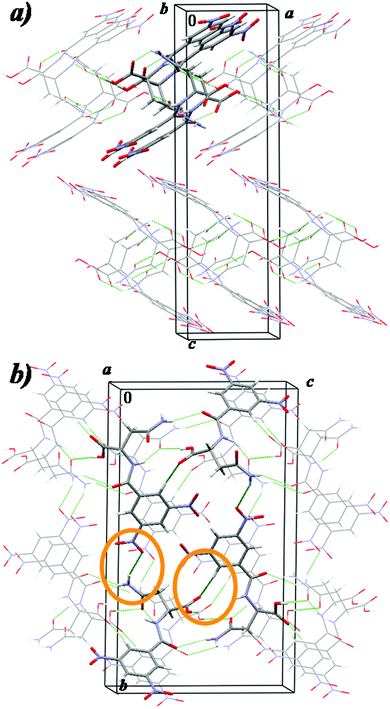 | ||
| Fig. 2 Packing of a) enantiomeric and b) racemic N-(3,5-dinitrobenzoyl)asparagine (C – grey, H – white, N – blue, O – red). | ||
Contrary to the case of DNBN-e, no separation of the hydrophobic from the hydrophilic parts is observed in DNBN-rac. Moreover, in DNBN-rac, the carbonyl O atom of the carboxylic group is involved in C–H⋯O hydrogen bonds only and the N atom of the β-amide group forms two hydrogen bonds: one (with the α-amide O atom) is very angular and the acceptor of the other is the nitro O atom (Fig. 2b and Table S2 in the ESI†). Taking into account that in DNBN-e, each potential donor of a strong hydrogen bond is involved in a suitable hydrogen bond, the above mentioned interactions in DNBN-rac would be rather surprising. However, the calculated density of DNBN-rac is greater than the calculated density of DNBN-e. This shows that the main force driving the racemate crystallization is achieving the most dense packing. It is worth adding that DNBN-rac and DNBN-e reveal similar thermal behavior (Fig. S5, S6 and S12 in the ESI†). The melting point of DNBN-rac is only 1 K higher than the melting point of DNBN-e (467 K).
The above results suggest that the presence of brucine or strychnine induces the formation of the self-assemblies of the N-(3,5-dinitrobenzoyl)-D- or N-(3,5-dinitrobenzoyl)-L-asparaginate anions stabilized by a set of hydrogen bonds. This is accomplished by hydrophobic (not necessarily chiral) interactions with the 3,5-dinitrobenzoyl group. It seems that introducing a factor which could increase the ability of the N-(3,5-dinitrobenzoyl)asparagine molecules for self-recognition by strong hydrogen bond formation may lead to the precipitation of a crystalline form similar to the enantiomeric one. In this case, molecules of the same enantiomer would be linked to each other by a set of hydrogen bonds mediated by the supramolecular synthon SS-N1. If the neighboring layers were homochiral, then the crystalline sample would be a conglomerate and generally, a result of spontaneous separation. If the neighboring layers were heterochiral, then the crystals would be polar. Both cases are rare and worth examination.
Introducing a factor which could increase the ability of the N-(3,5-dinitrobenzoyl)asparagine molecules for self-recognition by strong hydrogen bond formation can be realized, for example, by decreasing the solvent–solute interaction strength. In further experiments, alcohols of different carbon chain lengths were used as solvents. Afterwards, the experiments were extended on solvents of different dielectric constants. Similar to the crystallizations of N-(3,5-dinitrobenzoyl)asparagine from aqueous solution, the crystallizations of N-(3,5-dinitrobenzoyl)asparagine from the alcohols and other above mentioned solvents were performed at room temperature by solvent evaporation (more details in the ESI†). Crystals precipitating from a 2-methylpropan-1-ol solution containing racemic N-(3,5-dinitrobenzoyl)asparagine belong to the orthorhombic space group P212121 and contain one enantiomer of the asparagine derivative in the asymmetric unit, which implies a spontaneous racemic resolution of N-(3,5-dinitrobenzoyl)asparagine (DNBN-srr).‡14–16 Similar results were achieved when ethyl acetate, propan-1-ol or butan-1-ol were used as solvents (generally from solvents of a low dielectric constant). When methanol or nitromethane were applied (solvents of a high dielectric constant), DNBN-rac precipitated. Crystallization from acetone or ethanol solutions afforded a mixture of both DNBN-rac and DNBN-srr (Fig. 3).
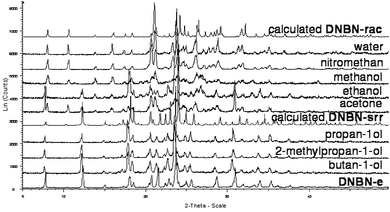 | ||
| Fig. 3 Comparison of the experimental and calculated PXRD patterns for N-(3,5-dinitrobenzoyl)asparagine precipitating from a given solvent containing a racemic (except for one enantiomeric, marked as DNBN-e) asparagine derivative. | ||
DNBN-srr is, in general, almost identical to DNBN-e. However, there are some small differences in their cell dimensions and consequently, in their densities and also in the geometry of the hydrogen bonds observed in both crystalline products. It is worth mentioning that the density of DNBN-srr is lower than the density of DNBN-e. The differences between the orthorhombic crystals obtained from a solution containing racemic or enantiomeric N-(3,5-dinitrobenzoyl)asparagine likely result from racemic twinning of the crystals obtained from the racemate. The racemic twinning is also manifested by the thermal behavior of the compound. The melting point of DNBN-srr obtained from butan-1-ol is 7 K lower than the melting point of DNBN-e and 8 K lower than the melting point of DNBN-rac (Fig. S5, S6, S7 and S12 in the ESI†). Since the racemic twinning results in the density and melting point lowering of the conglomerate, we wondered whether the conglomerate recrystallization deepens these effects or leads to a crystalline compound whose density and stability is more similar to the enantiomeric one. Preliminary results display that recrystallization from butan-1-ol causes a further lowering of the melting point of about 1.43 K (Fig. S5 and S8 in the ESI†). Other preliminary experiments show that as the dielectric constant is higher, the melting point of the resulting conglomerate is higher too and thus more similar to the crystals of the enantiomeric compound. Since the solvents dielectric constant depends on the temperature, it is likely that a suitable selection of crystallization temperatures can be another factor which allows for the crystallization of the racemate or conglomerate and also allows for tuning of the melting point of the conglomerate.
The above results unambiguously show that, depending on the dielectric constant of the solvent used for crystallization, racemic N-(3,5-dinitrobenzoyl)asparagine undergoes spontaneous separation. In turn, the different densities and the different thermal behavior of the conglomerate and of the pure enantiomer indicate racemic twinning in the conglomerate. Taking into account the layered structure of the conglomerate and the fact that consecutive layers are linked to each other by N⋯O interactions between the nitro groups, it is possible that the neighboring layers are heterochiral, which can justify the racemic twinning. On the other hand, the possibility of the presence of heterochiral layers generates another question, whether it is possible to obtain crystals in which each layer is bonded to a layer of the opposite enantiomer. Such crystals would likely belong to the mm2 point group and would be polar. The lower melting point of the conglomerate in comparison to the crystals of the pure enantiomer indirectly shows that each heterochiral connection in the enantiomorphous crystals succeeds in lowering the stability. Thus, if each layer were linked to layers of the opposite enantiomer, it could significantly influence the stability of the resulting crystals.
Precipitation of the conglomerate depending on the dielectric constant also gives an insight into the mechanism of racemic resolution by applying a hydrophobic resolving agent, such as brucine or strychnine. Similar to the effect of a solvent of a lower dielectric constant, the presence of brucine or strychnine increases the ability of the asparagine derivative for self-recognition by hydrogen bond formation.
It is worth mentioning that the lattice energy is the main criterion in techniques of crystal structure prediction to predict whether a chiral compound should resolve spontaneously.22 It seems that the conglomerate under investigation should not crystallize because of its lower melting point and lower density than the melting point and density of the racemic crystals. However, the conglomerate precipitated because of the attractive interactions formed thanks to the suitable solvent properties. It shows the remarkably important role that the solution properties can play (the dielectric constant of the solvent, ionic strength, presence of additives etc.), leading to a change in the nature of the molecular recognition. In turn, the change in the nature of the molecular recognition can have serious consequences for living as well as artificial systems. Information on intermolecular interactions can allow us to resolve spontaneously “unresolved” chiral compounds and can facilitate the synthesis of suitable polymorphs predicted in techniques of crystal structure prediction.
Acknowledgements
We thank the Ministry of Science and Higher Education of Poland for their financial support and Grant No. 1486/M/WCH/11.Notes and references
- (a) R. Fasel, M. Parschau and K.-H. Ernst, Nature, 2006, 439, 449 CrossRef CAS; (b) I. Weissbuch, M. Berfeld, W. Bouwman, K. Kjaer, J. Als-Nielsen, M. Lahav and L. Leiserowitz, J. Am. Chem. Soc., 1997, 119, 933 CrossRef CAS; (c) M. Böhringer, K. Morgenstern, W.-D. Schneider and R. Berndt, Angew. Chem., Int. Ed., 1999, 38, 821 CrossRef; (d) M. Stöhr, S. Boz, M. Schär, M.-T. Nguyen, C. A. Pignedoli, D. Passerone, W. B. Schweizer, C. Thilgen, T. A. Jung and F. Diederich, Angew. Chem., Int. Ed., 2011, 50, 9982 CrossRef.
- J. Jacques, A. Collet and S. H. Wilen, Enantiomers, Racemates and Resolutions, Krieger Publishing Company, Malabar, FL, 1991 Search PubMed.
- C. P. Brock, W. B. Schweizer and J. D. Dunitz, J. Am. Chem. Soc., 1991, 113, 9811 CrossRef CAS.
- (a) L. Pérez-García and D. B. Amabilino, Chem. Soc. Rev., 2007, 36, 941 RSC; (b) G. Coquerel, Top. Curr. Chem., 2007, 269, 1 CrossRef CAS.
- L. Pasteur, Ann. Chim. Phys., 1848, 24, 442 Search PubMed.
- I. Ostromisslensky, Ber. Dtsch. Chem. Ges., 1908, 41, 3035 CrossRef.
- (a) S. Kojo and K. Tanaka, Chem. Commun., 2001, 1980 RSC; (b) S. Kojo, H. Uchino, M. Yoshimura and K. Tanaka, Chem. Commun., 2004, 2146 RSC.
- (a) L. Addadi, Z. Berkovitch-Yellin, I. Weissbuch, J. van Mil, L. J. W. Shimon, M. Lahav and L. Leiserowitz, Angew. Chem., Int. Ed. Engl., 1985, 24, 466 CrossRef; (b) I. Weissbuch, L. Addadi, M. Lahav and L. Leiserowitz, Science, 1991, 253, 637 CAS.
- (a) I. Wessbuch, L. Leiserowitz and M. Lahav, Top. Curr. Chem., 2005, 259, 123 CrossRef; (b) R. Noyori, Angew. Chem., Int. Ed., 2002, 41, 2008 CrossRef CAS; (c) W. S. Knowles, Angew. Chem., Int. Ed., 2002, 41, 1999 CrossRef; (d) K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2024 CrossRef CAS.
- (a) R. O. Gould and M. D. Walkinshaw, J. Am. Chem. Soc., 1984, 106, 7840 CrossRef CAS; (b) A. Białońska and Z. Ciunik, CrystEngComm, 2006, 8, 66 RSC; (c) A. Białońska and Z. Ciunik, CrystEngComm, 2006, 8, 640 RSC; (d) A. Białońska and Z. Ciunik, CrystEngComm, 2007, 9, 570 RSC; (e) A. Białońska and Z. Ciunik, Acta Crystallogr., Sect. B: Struct. Sci., 2006, B62, 1061 Search PubMed.
- A. Białońska and Z. Ciunik, CrystEngComm, 2011, 13, 967 RSC.
- (a) I. Kalf, R. Wang and U. Englert, J. Organomet. Chem., 2006, 691, 2277 CrossRef CAS; (b) S. Larsen and H. Lopez de Diego, Acta Crystallogr., Sect. B: Struct. Sci., 1993, B49, 303 CrossRef CAS; (c) M.-C. Brianso, M. Leclercq and J. Jacques, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1979, B35, 2751 CrossRef; (d) H. Lopez de Diego, Acta Chem. Scand., 1994, 48, 306 CrossRef CAS; (e) K. Kinbara, Y. Hashimoto, M. Sukegava, H. Nohira and K. Saigo, J. Am. Chem. Soc., 1996, 118, 3441 CrossRef CAS; (f) O. Achmatowicz, I. Malinowska, B. Szechner and J. K. Maurin, Tetrahedron, 1997, 53, 7917 CrossRef CAS; (g) A. Białońska and Z. Ciunik, Cryst. Growth Des., 2013, 13, 111 CrossRef.
- J. T. Wróbel, Preparatyka i Elementy Syntezy Organicznej, PWN, Warszawa, 1983 Search PubMed.
- CrysAlis ‘RED’, Oxford Diffraction Ltd., Abingdon, Oxfordshire, England, 2009 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, A64, 112 CrossRef.
- XPREP – Data Preparation & Reciprocal Space Exploration, Ver. 5.1/NT, Bruker Analytical X-ray System, 1997 Search PubMed.
- J. H. Robertson and C. A. Beevers, Acta Crystallogr., 1951, 4, 270 CrossRef CAS.
- F. Toda, K. Tanaka, H. Ueda and T. Oshima, Isr. J. Chem., 1985, 25, 338 CAS.
- W. Humphrey, A. Dalke and K. Schelten, J. Mol. Graphics, 1996, 14, 33 CrossRef CAS.
- H. Mubashar-ur-Rehman, I. U. Khan, M. N. Arshad and K. T. Holman, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, E66, o2596 Search PubMed.
- M. N. Arshad, H. Mubashar-ur-Rehman, I. U. Khan, M. Shafiq and K. M. Lo, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, E66, o541 Search PubMed.
- (a) J. Kendrick, M. D. Gourlay, M. A. Neumann and F. J. J. Leusen, CrystEngComm, 2009, 11, 2391 RSC; (b) M. D. Gourlay, J. Kendrick and F. J. J. Leusen, Cryst. Growth Des., 2007, 7, 56 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental part, 1H NMR, IR, DTA-TG, CCDC 933059–933066. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ce40778k |
| ‡ Crystal data for DNBN-rac: C11H10N4O8, M = 326.23, monoclinic, P21/c, a = 4.583(2), b = 21.947(5), c = 12.852(3) Å, β = 92.76(2)°, V = 1291.2(7) Å3, Z = 4, Dc = 1.678 Mg m−3, T = 100(2) K, R = 0.064, wR = 0.145 (710 reflections with I > 2σ(I)) for 208 variables, CCDC 933066. DNBN-e: C11H10N4O8, M = 326.23, orthorhombic, P212121, a = 6.371(2), b = 9.260(2), c = 22.629(4) Å, V = 1335.0(6) Å3, Z = 4, Dc = 1.623 Mg m−3, T = 100(2) K, R = 0.042, wR = 0.086 (2185 reflections with I > 2σ(I)) for 208 variables, CCDC 933064. DNBN-srr: C11H10N4O8, M = 326.23, orthorhombic, P212121, a = 6.381(2), b = 9.276(2), c = 22.660(3) Å, V = 1341.2(5) Å3, Z = 4, Dc = 1.616 Mg m−3, T = 100(2) K, R = 0.068, wR = 0.165 (1844 reflections with I > 2σ(I)) for 208 variables, CCDC 933065. |
| This journal is © The Royal Society of Chemistry 2013 |
