Comprehensive determination of the solid state stability of bethanechol chloride active pharmaceutical ingredient using combined analytical tools†
Abstract
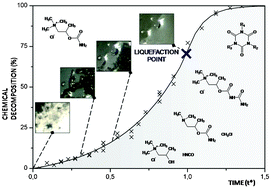
The use of an integrative analytical approach allowed us to establish the intrinsic solid state stability of bethanechol chloride (BC), an active pharmaceutical ingredient used in the treatment of urinary retention. First, the crystal structure of the monoclinic form has been described using single crystal X-ray diffraction studies. Second, thermal analyses revealed that the compound degrades upon melting, with an apparent melting temperature estimated to be 231 °C. No transition from the monoclinic to the orthorhombic form has been observed, suggesting that the monoclinic form is the stable one. Third, the two-step melting–decomposition process has been elucidated by liquid chromatography and thermogravimetry coupled to mass spectrometry. The first step corresponds to the sample liquefaction, which consists of the gradual dissolution of bethanechol chloride in its liquid degradant, i.e. betamethylcholine chloride. This step is in agreement with Bawn kinetics and the activation energy of the reaction has been estimated at 35.5 kcal mol−1. The second step occurs with accelerated degradation in the melt. Elucidation of secondary decomposition pathways evidenced autocatalytic properties conferred by the formation of both isocyanic acid and methyl chloride. Finally, dynamic water vapor sorption analysis showed a substantial hygroscopicity of the drug substance. A deliquescent point has been determined at 56% relative humidity at 25 °C.

 Please wait while we load your content...
Please wait while we load your content...