 Open Access Article
Open Access ArticleOn the effect of silicon in CVD of sp2 hybridized boron nitride thin films
Mikhail
Chubarov
*,
Henrik
Pedersen
,
Hans
Högberg
and
Anne
Henry
Department of Physics, Chemistry and Biology, Linköping University, SE-581 83 Linköping, Sweden. E-mail: mihails.cubarovs@liu.se; Fax: +46 13 28 8983; Tel: +46 13 13 7568
First published on 8th November 2012
Abstract
The influence of silicon on the growth of epitaxial rhombohedral boron nitride (r-BN) films deposited on sapphire (0001) by chemical vapor deposition is investigated. X-ray diffraction measurements and secondary ion mass spectrometry show that silicon favors the formation of r-BN and is incorporated into the film.
Today, there is a growing interest in hexagonal (h-BN) and/or rhombohedral (r-BN) thin films. This is due to the outstanding physical and chemical properties of BN with suggested applications either as microelectronic or optoelectronic devices. These sp2 hybridized phases of BN differ only by the stacking sequence of the basal planes (ABAB… for h-BN and ABCABC… for r-BN) and they exhibit many interesting properties such as a wide band gap of about 6 eV, high thermal and chemical stability and negative electron affinity.1,2
In a recent work we reported that r-BN can be epitaxially grown by thermally-activated chemical vapor deposition (CVD) on sapphire (Al2O3) (0001) substrates, using an aluminum nitride (AlN) buffer layer.3 The results showed that the growth of high quality films is restricted to a narrow process window at a temperature close to 1500 °C, using a pressure in the 70–100 mbar range and N/B ratio between 600 and 700. For the nucleation a thin and strained AlN buffer layer is required.4
To modify the semiconductor properties of sp2-BN films, silicon doping offers an interesting route since the element has been proposed as a n-type dopant for sp3-BN and is widely used as n-type dopant in other III-N materials.5–8 For example, Ronning et al.9 reported on the influence of Si doping on the crystalline quality of cubic sp3-BN (c-BN). They found that the amount of incorporated sp2-BN phase into the c-BN crystal increased when the amount of Si was increased in the gas phase. When the Si concentration in the film was higher than 4%, 100% sp2-BN was obtained.9 Further, the effect of Si on the crystalline structure of c-BN has been confirmed by other groups.10,11 These studies suggest that Si mainly interacts with nitrogen in the stabilization of sp2-BN. This conclusion is based on the formation of Si–N bonds, which are easily observed as a characteristic peak at 840 cm−1 using Fourier Transform Infrared (FTIR) spectroscopy even for films with Si concentrations of ∼1%.8,10–13 However, to the best of our knowledge there is no study on the influence of Si on the CVD grown sp2-BN crystal structure and its electrical properties.
In this work, we show that Si stabilizes the growth of r-BN by CVD at a temperature of 1500 °C and a pressure of 70 mbar, using a precursor mixture of triethyl boron ((C2H5)3B, TEB) and ammonia (NH3) in hydrogen (H2) carrier gas with or without the addition of silane (SiH4).
The growth of the sp2-BN films was conducted in a CVD reactor with a hot-wall design and a graphite susceptor, i.e. reaction cell, coated either by silicon carbide (SiC) or tantalum carbide (TaC). The susceptor is packed into a graphite isolation, placed in a quartz tube and heated inductively by an rf coil around the quartz tube. As substrate material, c-axis oriented sapphire was used. TEB of semiconductor grade and ammonia (99.999%) were used as boron and nitrogen precursors, respectively. Silane (99.999%) diluted to 200 ppm in H2 (99.9996%) was used as silicon source. The optimal ratio of Si/B was 0.03 when adding SiH4 to the process gases. Prior to the sp2-BN growth, an AlN buffer layer was formed on the substrate by nitridation with ammonia at high temperature (1300–1500 °C).3 The growth experiments were conducted at a temperature of 1500 °C and at a pressure of 70 mbar, using H2 carrier gas, nitrogen-to-boron (N/B) ratio of 600–700, and boron concentration in the gas phase (B/H2) of 0.013%; further details on the growth process can be found in ref. 4.
X-ray diffraction (XRD) measurements in the Bragg–Brentano geometry were performed in a PANalytical X'Pert PRO powder diffractometer using Cu Kα1 radiation. XRD ω-scans were measured in a PANalytical EMPYREAN MRD with Cu Kα1 radiation, hybrid mirror and parallel plate collimator as primary and secondary optics, respectively. Pole figure measurements were carried out in a Philips X'Pert MRD, applying Cu Kα1 radiation, crossed slits as primary optics and parallel plate collimator on the detector side. In all types of diffraction analysis Cu Kβ radiation was removed by a Ni filter.
Fourier transform infrared (FTIR) spectrum measurements were done using a BioRad QS 2200 Fourier Transform Infrared Spectrometer equipped with a potassium bromide (KBr) beam splitter and a deuterated triglycine sulfate (DTGS) detector. Measurements were conducted at room temperature using a glowbar light source (incidence angle of 30°), 4 cm−1 resolution and averaged over 10 scans.
Secondary ion mass spectrometry (SIMS) measurements were performed by Evans Analytical Group (EAG) East Windsor, New Jersey, USA by sputtering material with Cs+ ions and collecting negative ions. The sputtering crater size was 100 × 100 μm2. Silicon, carbon and oxygen were the investigated elements and their detection limit was 1 × 1016 atoms cm−3 for silicon and 5 × 1017 atoms cm−3 for carbon and oxygen.
The SiC protective coating of the graphite susceptor was found to be significantly etched by the process gases, particularly NH3. When the coating was removed, the graphite was exposed and the etching process was accelerated. This resulted in an involuntary release of impurities as well as dramatic changes in the temperature distribution in the reaction cell. To avoid such process damaging effects, we used a TaC coated graphite susceptor as this coating material is commonly applied in the growth of other III-N semiconductor materials.
Depositions performed with the TaC coated susceptor did not result in the growth of high quality r-BN, albeit using the same growth conditions (gas flows, temperature, and pressure) as for the SiC coated susceptor. XRD revealed that the grown BN films were the less ordered form of sp2-BN – turbostratic BN (t-BN) or even amorphous. However, an interesting observation was that growth experiments performed directly after a silicon melting test used for temperature calibration resulted in the growth of high quality r-BN film, but with a decrease in the quality already for the next deposition in the series as confirmed by XRD from the growth of t-BN films. This observation triggered a hypothesis that either ammonia and/or hydrogen etching of the SiC coating on the susceptor or evaporated Si from the melt test could release low amount of Si that could be incorporated in the film to yield high quality r-BN. To test this hypothesis, growth experiments with SiH4 added to the gas mixture were performed, using the TaC coated susceptor.
Formation of high quality r-BN could be observed in the TaC coated susceptor when a low amount (Si/B = 0.03) of SiH4 was added to the gas mixture during growth using identical growth parameters as used in the SiC coated susceptor (T = 1500 °C, p = 70 mbar, N/B = 614, B/H2 = 0.013%). Additional adjustments of the N/B ratio from 614 to 640 further improved the crystalline quality of the r-BN films.
Fig. 1 illustrates X-ray diffractograms of BN film grown with a SiC coated susceptor (N/B = 614) (red, A), with the TaC coated susceptor (N/B = 640) without addition of SiH4 (green, B), and with the TaC coated susceptor with addition of SiH4 (Si/B = 0.03, N/B = 640) (blue, C). As evident, r-BN films are only formed in the SiC coated susceptor and in the TaC coated susceptor when SiH4 is added. To investigate the crystalline quality of the grown films XRD ω-scan measurements of the r-BN 0003 peak was carried out. XRD ω-scan measurements confirmed formation of r-BN when SiH4 is added to the gas mixture showing no peak in range from 12° to 15° in omega at 2θ angle of 26.125° for the films grown without addition of SiH4, whereas for the samples grown with addition of SiH4 the ω-scan XRD measurements showed a peak with a full width at half maximum of 1.07° at a 2θ angle of 26.716°. The 2θ angles are different in both cases since ω-scans should be done at the maximum intensity of the peak under investigation. This observation proves the increase in the crystal quality of the grown film when SiH4 is added to the gas mixture during the growth.
 | ||
| Fig. 1 X-ray diffractograms of boron nitride films grown in the SiC coated susceptor (red line, A), in the TaC coated susceptor (green line, B) and in the TaC coated susceptor with addition of SiH4 during the growth (blue, C). The r-BN peaks are present only when Si is present in the reactor, either as SiC coating or as gas additive. The features observed in the range 44–46° are due to the sample holder, and the small peak at 37.51° is the 0006 peak of sapphire from the diffraction of the Cu Kβ line. | ||
Epitaxial growth of r-BN was confirmed by pole figure measurements of the films grown in the TaC coated susceptor with addition of SiH4 to the gas phase. Further, the measurements reveal a twinned r-BN structure consistent with our previous observations.3,4
FTIR measurements of the sp2-BN films grown in the TaC coated susceptor, with and without addition of silane, showed two sp2-BN characteristic phonon lines around 810 cm−1 and 1350 cm−1 which correspond to the out-of-plane bending vibrations of B–N–B and the in plane B–N stretching vibrations respectively (Fig. 2).11 The phonon line at around 840 cm−1 associated with Si–N bonds was not observed in any of the recorded spectra.12,13 It should be noted that the FTIR spectra recorded from the films grown with or without SiH4 addition are almost identical whereas the crystalline structure of these films are completely different, see (Fig. 1). The weak line seen at 950 cm−1 in the FTIR spectra is caused by the system response due to the rapid decrease of the signal. Such characteristics are observed also for sapphire and SiC substrates in the used spectrometer.
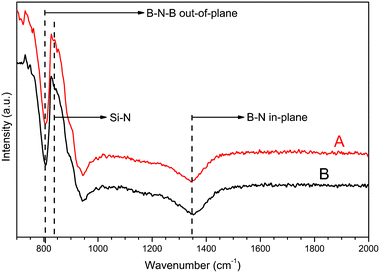 | ||
| Fig. 2 FTIR spectra of sp2-BN films grown without (red line, A) and with (black line, B) SiH4 in the gas mixture during deposition. The position where a peak from Si–N bonds is expected is marked in the figure. Peak at 950 cm−1 is due to the system response and is not attributed to the material. | ||
The absence of Si–N bonds in the FTIR spectrum suggests low concentration of Si in the films, i.e. much less than the ∼1% detected by the technique in the reported studies.8,10–13 SIMS measurements showed that Si is present in both types of films (Fig. 3). However, the average concentration of Si was found to be 1 × 1019 cm−3 and 4 × 1019 cm−3 for the films grown without and with SiH4 addition to the gas phase during growth, respectively. Assuming an ideal r-BN density this yields a Si to B ratio of 0.02% and 0.07% for both films. This supports the FTIR observation that Si is present as a trace element in the films and with an average concentration of ∼0.01 to 0.03% assuming an atomic density of ∼1 × 1023 cm−3 for bulk r-BN. In both cases, a gradient of the Si concentration through the whole film is observed with a larger slope for the last 20 nm interpreted as Si accumulation on the surface (see Fig. 3). Both investigated films were about 200 nm thick. In the case of the sample grown without addition of Si, the Si concentration ranges from 4 × 1018 to 2 × 1019 cm−3 and reaches a value of 5 × 1019 cm−3 at the surface. For the film grown with SiH4 addition, the Si concentration changes from 3 × 1019 to 6 × 1019 cm−3 and increases up to 1 × 1020 cm−3 at the surface (Fig. 3). About 1 × 1021 cm−3 oxygen atoms were uniformly incorporated throughout the films thickness, however with a slight increase to 2 × 1021 cm−3 for the last 20 nm. For the C atoms an increasing concentration was observed from 2 × 1020 to 5 × 1020 cm−3 through the bulk of the films, and with a increase to 8 × 1020 cm−3 at the surface during the last 20 nm to the surface. In both cases (O and C incorporation) the same profile was obtained for the film grown with or without addition of SiH4. Such SIMS profiles are in agreement with previous reported impurities concentrations obtained with time-of-flight elastic recoil detection analysis (ToF ERDA)4 where a highest concentration of O and C atoms was found at the top surface of the BN layers. In addition, higher concentration of both O and C atoms were reported when the grown films were with a turbostratic phase (t-BN). This suggests that both C and O atoms hamper the nucleation of r-BN and could deteriorate the crystalline quality at too high concentration. In contrast, silicon atoms act on the surface and desorb from the surface leading to a lower concentration in the film. Moreover, the results suggest that Si helps to stabilize the r-BN lattice. A possible explanation for the stabilizing effect of Si on the r-BN crystal structure is that the silicon atom with its extra valence electron can stabilize the structure by either being intercalated between basal plans or by replacing boron in the lattice; the latter type of positioning is considered since boron and silicon exhibit more similar electronegativity values and atomic radii when compared to nitrogen. The presence of Si atoms in the film grown without addition of SiH4 could be explained by a contamination of the susceptor walls from previous doped growth runs and then an evaporation of the Si atoms from the reactor cell walls during the growth. However, being in too low concentration its effect on the stabilization of the r-BN phase is minimized. In addition, the incorporation efficiency for Si in r-BN is found to be rather low; by comparing the concentration of SiH4 used during the growth and the amount of Si atoms deduced from SIMS in the film, only about 2% of added Si atoms are incorporated into the film.
 | ||
| Fig. 3 SIMS spectra of sp2-BN films grown without (red line, A) and with (black line, B) SiH4 in the gas mixture during deposition. | ||
Conclusions
The results presented in this study suggest that Si atoms have a positive impact on the quality of BN films grown by thermally-activated CVD. Addition of small amount of SiH4 to the gas mixture during sp2-BN deposition improves the crystalline quality of the growing film and contributes to the growth of high quality epitaxial r-BN instead of less ordered t-BN film. SIMS measurements suggest that Si acts as surface active material which stimulates the formation of high quality r-BN. Possible mechanism for the stabilization of the r-BN lattice based on Si acting as electron donor is suggested together with possible position of Si in the r-BN lattice. The incorporation efficiency is found to be rather low with an accumulation on the surface.Acknowledgements
The Swedish Research Council (VR 621-2009-5264 and VR 622-2008-1247) is gratefully acknowledged for financial support.References
- T. Taniguchi and K. Watanabe, J. Cryst. Growth, 2007, 303, 525 CrossRef CAS
.
- C. Kimura, K. Okada, S. Funakawa, S. Sakata and T. Sugino, Diamond Relat. Mater., 2005, 14, 719 CrossRef CAS
.
- M. Chubarov, H. Pedersen, H. Högberg, V. Darakchieva, J. Jensen, P. O. Å. Persson and A. Henry, Phys. Status Solidi RRL, 2011, 5, 397 CrossRef CAS
.
- M. Chubarov, H. Pedersen, H. Högberg, J. Jensen and A. Henry, Cryst. Growth Des., 2012, 12, 3215 CAS
.
- S. B. Thapa, J. Hertkorn, F. Scholz, G. M. Prinz, R. A. R. Leute, M. Feneberg, K. Thonke, R. Sauer, O. Klein, J. Biskupek and U. Kaiser, J. Cryst. Growth, 2008, 310, 4939 CrossRef CAS
.
- K. S. Kim, C. S. Oh, W.-H. Lee, K. J. Lee, G. M. Yang, C.-H. Hong, E.-K. Suh, K. Y. Lim, H. J. Lee and D. J. Byun, J. Cryst. Growth, 2000, 210, 505 CrossRef CAS
.
- M. Higashiwaki, T. Inushima and T. Matsui, Phys. Status Solidi B, 2003, 240, 417 CrossRef CAS
.
- J. Ying, X. W. Zhang, Z. G. Yin, H. R. Tan, S. G. Zhang and Y. M. Fan, J. Appl. Phys., 2011, 109, 023716 CrossRef
.
- C. Ronning, A. D. Banks, B. L. McCarson, R. Schlesser, Z. Sitar, R. F. Davis, B. L. Ward and R. J. Nemanich, J. Appl. Phys., 1998, 84, 5046 CrossRef CAS
.
- J. Ying, X. W. Zhang, Y. M. Fan, H. R. Tan and Z. G. Yin, Diamond Relat. Mater., 2010, 19, 1371 CrossRef CAS
.
- S. Kurooka, T. Ikeda and A. Tanka, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 206, 1088 CrossRef CAS
.
- W. R. Knolle and J. W. Osenbach, J. Appl. Phys., 1985, 58, 1248 CrossRef CAS
.
- G. Beshkov, S. Lei, V. Lazarova, N. Nedev and S. S. Georgiev, Vacuum, 2002, 69, 301 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |
