Highlights from the 48th EUCHEM conference on stereochemistry, Bürgenstock, Switzerland, May 2013
Gonçalo J. L.
Bernardes
*ab
aDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: gb453@cam.ac.uk
bInstituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal. E-mail: gbernardes@fm.ul.pt
First published on 13th August 2013
Recognizing known faces of possible speakers at the train station is usually how the journey to the outstanding Bürgenstock conference starts, now held in the majestic village of Brunnen, Switzerland (Fig. 1). Tradition dictates that the programme remains a mystery until the beginning of the conference and that speakers can only give one plenary lecture during their life-time. The conference is characterized by a well-balanced blend of scientists from both academia and industry and participants (roughly 100) are requested to stay for the whole week. Every year since the 1st edition back in 1965, the Bürgenstock gathers 14 of the world's leading scientists within the chemical sciences to present their research under the inspirational views of the Swiss Alps (Fig. 1). | ||
| Fig. 1 Panoramic picture of the inspirational Brunnen. Photo credit: Martina Steiner. | ||
President Luisa de Cola (Université de Strasbourg and Karlsruhe Institute of Technology) together with the local organising committee constituted of Alain De Mesmaeker (Syngenta Crop Protection Research, Stein), Jérôme Lacour (University of Geneva), Reto Naef (Novartis Pharma AG, Basel), Philippe Renaud (University of Bern) and Helma Wennemers (ETH Zürich) undertook the challenging job of putting together the scientific programme of the 2013 edition as well as an entertaining set of social activities. While stereochemistry was at the centre of the Bürgenstock meeting, today's scientific program covers a wide range of fundamental aspects within the chemical sciences as well as interdisciplinary approaches that, importantly, are providing a path toward collectively addressing the many challenges of chemistry. These efforts were translated into a diverse and rich scientific program, the highlights of which are described in this report.
The President opened the conference by welcoming the participants and presenting the Guest of Honor of the 48th EUCHEM conference on stereochemistry, David Reinhoudt (Twente University). This was followed by highlights of the conference procedures and the promise of chocolate during the lectures, given the President is a confessed chocolate lover. Of course, the President offered the responsibility for the weather to the Vice-President Antonio Echavarren (Institute of Chemical Research of Catalonia), who did a great job keeping the rain away from Brunnen during most of the conference. The conference had officially started and a banquet dinner preceded the first evening lecture.
The scientific program started with a fascinating lecture by Michael Grätzel (EPFL Lausanne) after an original introduction involving “explosions and flames” by Katharina Fromm (University of Fribourg). The quest for cleaner energy and more efficient energy storage methods is likely one of the major challenges our world faces. Michael Grätzel pioneered the development of energy conversion systems by using mesoscopic materials. He took us on an incredible journey starting with the first prototype of a dye-sensitized nanocrystal cell (DSC) in 1988 and its publication in 19911 to today's integrated photovoltaic glass panels. By covering the surface of mesoscopic oxide films with an appropriate molecular chromophore it is possible to efficiently harvest and convert sunlight into electricity. With today's current improved DSC, solar-to-electric power-conversion efficiency (PCE) under full sunlight can reach values above 13%.2
The second day of the Bürgenstock meeting began with two lectures on materials bioengineering. Jeffrey Hubbel (EPFL Lausanne) demonstrated on how materials and proteins may be engineered to precisely modulate cellular responses.3 The power of these strategies were underscored by impressive applications in tissue engineering and immunology. For example, a simple and efficient system was designed and constructed by engineering the growth factor microenvironment with fibronectin domains that resulted in wound and bone tissue healing.4 In the immunotherapeutics context, small antigen modified nanoparticles were shown to effectively target antigen-presenting cells after pulmonary administration. This led to induction of a potent immune response in the lung and at other mucosal surfaces, conferring protection against viral infections such as influenza.5
Still on materials engineering, Heather Maynard (UCLA) illustrated how to build biomimetic polymers and smart polymers for drug-delivery purposes. The inherent instability of basic fibroblast growth factor (bFGF), a protein that displays key roles in a number of essential cellular processes, during storage and delivery has limited its therapeutic application. By covalent conjugation with a heparin-mimicking polymer, a copolymer consisting of styrene sulfonate units and methyl methacrylate units bearing poly(ethylene glycol) side chains, it was possible to stabilize bFGF while retaining its biological activity, a significant step towards its clinical use.6 Furthermore, the Maynard research group has reported the synthesis of trehalose side chain polymers for stabilization of protein conjugates to environmental stressors. When conjugated to proteins, the trehalose polymers significantly increased stability toward lyophilization and heat while retaining the native protein activity. Finally, the glycopolymers were compared to equivalent concentrations of trehalose alone and poly(ethylene glycol) (PEG) and found to be superior at stabilizing the protein to lyophilization and heat.7
The afternoon brought an exciting poster session which was preceded by 5 short lectures that served as “appetizers”. Poster sessions at Bürgenstock are an excellent stage for discussion and interactions between junior and senior scientists, and academics and industry participants. The short lectures were presented by some of Europe's most promising young scientists: Beat Fierz (EPFL Lausanne), Matthew Fuchter (Imperial College), Franziska Schoenebeck (ETH Zürich), Jan Streuff (Albert-Ludwigs-Universität Freiburg) and Mariola Tortosa (Universidad Autonoma de Madrid).
The evening lecture was given by Dennis Curran (University of Pittsburgh) who shared is work on fluorous mixing synthesis for the construction of small-libraries of complex natural product stereoisomers in single form. By fluorous tagging of reactants, products can later be demixed based on the fluorine content. Recently, his research group has improved this technique through the use of binary encoding such that a total of four tags can be employed to label in a unique manner a library containing all 16 diastereomers of natural products macrosphelides A and E. While the structures of macrosphelides A and E are known, the library data enabled determination of the correct stereo-structure of macrosphelide D.8
The next morning was reserved to recent developments in C–C and C–F bond formation reactions. Michael Krische (University of Texas at Austin) gave a outstanding lecture on the formation of C–C bonds under hydrogenation and transfer hydrogenation conditions and how these were capitalised in the total synthesis of the challenging natural products 6-deoxyerythronolide B9 and bryostatin 7.10 Eight and five C–C bonds formed using hydrogenative methods were used for the synthesis of 6-deoxyerythronolide B and bryostatin 7, respectively. He also presented the impressive direct redox-triggered C–C coupling of alcohols and butadiene that results in the formation of carbonyl crotylation products with high levels of anti-diastereoselectivity and enantioselectivity in the absence of stoichiometric by-products (Fig. 2A).11 Advances in C–C bond forming reactions will significantly facilitate the access to and the biological evaluation of complex polyketides.
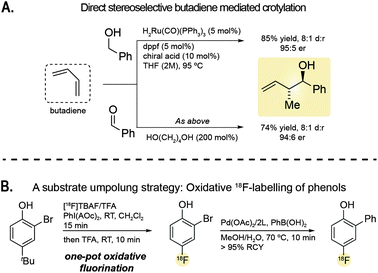 | ||
| Fig. 2 (A) Direct stereoselective butadiene mediated crotylation. (B) A substrate umpolung strategy for the or the radiochemical synthesis of aryl fluorides (ref. 13). L = 2-amino-4,6-dihydroxypyrimidine. | ||
The second lecture of the morning was presented by Véronique Gouverneur (University of Oxford) who gave an insightful overview of the importance of labeling strategies with the unnatural radionuclide 18F, which is particularly suited for use with imaging methods such as positron emission tomography (PET).12 While methods for fluorine incorporation are available and have become routinely used by medicinal chemists for the precise tuning and probing of the properties of small molecule binders, few such methods can be translated into “hot” conditions. Véronique Gouverneur presented major advances in her lab for the efficient incorporation of 18F into small molecules including, for example, a metal-free umpolung strategy that enables oxidative fluorination of electron-rich aromatic compounds using [18F]fluoride, with the oxidation event targeting the electron-rich substrate (aryl umpolung) rather than the [18F]fluoride (Fig. 2B).13 The method is rapid enough—18F has a half-life of 109.7 minutes—allowing subsequent palladium-mediated Suzuki–Miyaura coupling of 2-bromo-4-[18F]fluorophenol with benzylboronic acid. These methods and others are currently applied for the production of 18F labelled probes for (pre)clinical applications and to accelerate drug discover.12
After lunch, and with the afternoon free, many participants took the opportunity to hike the surrounding mountains with stunning views across Lake Lucerne and the Swiss Alps. And in accordance with tradition, there was no lecture scheduled for this evening, but instead an after-dinner musical concert by Pilar (vocals), Federico Ferrandina (guitar) and Andrea Colella (double bass) which was followed by a get-together with the musicians.
The morning of the fourth conference day led us into the world of materials science and began with a spectacular lecture by Christoph Weder (University of Fribourg) on the design of polymers with specific molecular parameters which confer specific macroscopic properties. In particular, the dynamic nature of non-covalent bonds has been explored to construct polymers that can be influenced in a predictable manner by exposure to predefined external stimuli. Christoph Weder exemplified the power of this approach when he discussed metallosupramolecular stimuli-responsive polymers in which scratch damages can be healed upon exposure to ultraviolet light (Fig. 3).14 He also presented recent developments on optically healable nanocomposites based on a telechelic poly(ethylene-co-butylene) functionalised with hydrogen-bonding ureidopyrimidone (UPy) groups at the termini, and cellulose nanocrystals decorated with the same binding motif.15 This design affords materials that display high stiffness and strength, and permit rapid and efficient optical healing.
 | ||
| Fig. 3 Schematic representation of optically healable supramolecular nanocomposites based on a telechelic poly(ethylene-co-butylene) functionalised with hydrogen-bonding ureidopyrimidone (UPy) groups at the termini, and cellulose nanocrystals decorated with the same binding motif. Also shown are AFM images of a deliberately damaged and an optically healed sample.15 Image courtesy of Mahesh Biyani, University of Fribourg. | ||
This was followed by a lecture from Gianluca Farinola (University of Bari), who described his research group's efforts in combining active organic and organometallic molecules and polymers with biological or inorganic nanostructures to build photo/electro-active architectures for applications ranging from organic photonics and electronics to biology. For instance, conjugation of polymers bearing a fluorinated backbone with organometallic complexes has resulted in electroluminescent materials with improved properties for organic light emitting diodes (OLEDs).16
The evening session saw a dramatic shift in focus from materials to enantioselective synthesis, with an insightful lecture by Huw Davies (Emory University) on carbenoid chemistry. The lecture started with an introduction of the center for selective C–H functionalisation (CCHF) (Fig. 4A). This pioneering initiative aims to promote joint efforts in the development of methods that enable selective functionalisation of traditionally “unactivated” C–H bonds and their use to accelerate programmes in drug discovery or materials science. This was followed by recent examples that resulted from collaborative work between different research groups at the CCHF. The first, from the groups of Huw Davies and Jin-Quan Yu (Scripps Research Institute), consisted of consecutive rhodium-catalysed enantioselective intermolecular C–H insertion followed by palladium-catalyzed C–H activation/C–O cyclisation to access 2,3-dihydrobenzofurans. These can then be further elaborated by a subsequent palladium-catalyzed intermolecular Heck-type sp2 C–H functionalisation to provide the core of the Lithospermic acid natural product (Fig. 4B).17 Additionally, combined synthetic and computational studies from the groups of Huw Davies and Djamaladdin Musaev (Emory University) not only showed that aryldiazoacetates and vinyldiazoacetates undergo highly enantioselective cyclopropanations with electron-deficient alkenes, but also provided key insights that may lead to novel rhodium-catalysed carbenoid reactions.18
 | ||
| Fig. 4 (A) Schematic representation of the structure of the center for selective C–H functionalization. Image courtesy of Huw Davies. (B) Consecutive C–H functionalization reaction for the enantioselective synthesis of elaborated 2,3-dihydrobenzofurans. | ||
Thursday's proceedings were dedicated to the field of chemical biology beginning with Nicolas Winssinger (University of Geneva) who gave an engaging overview of nucleic acids templated reactions and their use for programming self-assemblies in chemical biology. A number of methods are now available to access nucleic acid-tagged libraries and to select the most promising compounds within such libraries. The Winssinger group has focused on the development of PNA-tagged small molecules that are displayed on DNA templates (Fig. 5A).19,20 This strategy has shown valuable utility for screening purposes,20 but also to probe the optimal geometry in multivalent interactions. This was the case of a nucleic acid-encoded carbohydrate library that led to the identification of consensus DC-SIGN binder motif which when placed in a dendrimer shows enhanced binding to gp120's.21
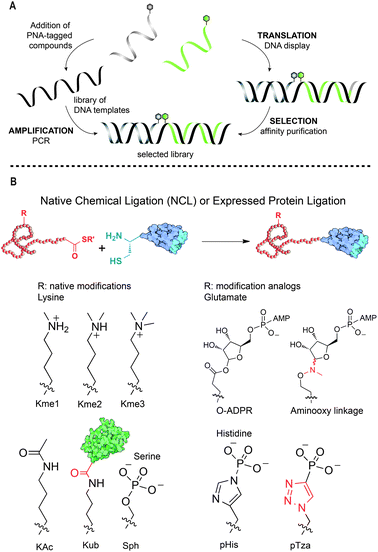 | ||
| Fig. 5 Two approaches in Chemical Biology: (A) schematic representation of DNA display of PNA-tagged molecules. Figure adapted from ref. 24. (B) Chemical approaches for the incorporation of PTMs and their analogs into histone proteins for the study of genetic regulation. Figure adapted from ref. 22. | ||
Subsequently, Tom Muir (Princeton University) described his fascinating work on chromatin, a complex of DNA and proteins that act as a dynamic signaling platform controlling gene function. Muir's group has expanded significantly the chemical tools available to install post-translational modifications (PTMs) and their analogs into pre-determined sites on histone proteins, the primary protein components of chromatin, enabling their study in genetic regulation.22Fig. 5B illustrates how to tracelessly link two synthetic peptides or a peptide and an expressed protein, respectively, with one of them bearing a pre-installed modification, by native chemical ligation (NCL) and expressed protein ligation (EPL). With this strategy many types of PTMs have been incorporated into histones enabling the study of their precise roles in genetic regulation.22 Impressive work has recently led to the discovery that pediatric diffuse intrinsic pontine gliomas (DIPGs) contain a missense K-to-M mutation leading to gain-of-function and inhibition of the enzymatic activity of Polycomb repressive complex 2 (PRC2). This result strongly supports that aberrant epigenetic silencing through H3K27M-mediated inhibition of PRC2 activity is responsible for promotion of gliomagenesis.23
Thursday afternoon was again reserved for 5 short lectures by young independent scientists: Gonçalo Bernardes (University of Cambridge and Instituto de Medicina Molecular, Lisbon), Micha Fridman (Tel Aviv University), Seiji Shirakawa (Kyoto University), Boris Vauzeilles (CNRS and Université Paris-Sud) and Sandeep Verma (Indian Institute Of Technology-Kanpur) which was followed by an exciting and intense poster session.
Viola Vogel (ETH Zürich) concluded the day by showing how her research group is decoding the underlying mechanisms behind bacteria and cell responses to mechanical stimuli and how these are converted into biochemical signals that can switch cell functions.25 As an example of a mechanical chemical switch, it was demonstrated that mechanical stretching of fibronectin fibres present in the extra-cellular matrix (ECM) are sufficient to physically destroy a cell-binding site providing insights on how the mechanobiology of ECM might regulate bacterial and cell-binding events during infection.26
This year's Vice-President and consequently the next President, Antonio Echavarren chaired the last section of the conference on Friday morning. First, Jun-Ichi Yoshida (Kyoto University) gave an insightful lecture on how to integrate organic synthesis on the basis of reactive intermediates to build organic molecules under flow chemistry.27 The approach is based on the so-called “cation-pool method” that consists of rapid generation of reactive organic cations in the absence of nucleophiles and subsequent reaction with nucleophiles before they decompose using an integrated flow microreactor system. This method enables, for instance, performing glycosylation reactions under flow28 and led to the development of a number of novel electrochemical organic reactions including electrochemical C–H amination29 for use in flow chemistry. The final scientific lecture was given by Bruno Chaudret (University of Toulouse) on the construction of organometallic-based nanoparticles and their uses.30 While still limited to a small number of organometallic precursors, Bruno Chaudret showed us examples of nano-objects built with precise control of both the growth and of the surface chemistry. With these particles of defined shapes, organisation and surface chemistry as well as physical properties are formed enabling their use in asymmetric catalysis and microelectronics.30
The president of the organising committee, Luisa de Cola, closed the meeting by thanking the lecturers and poster presenters for stimulating scientific presentations, the participants for contributing in exciting discussions, the colleagues of the organising committee for their assistance in the organisation, the students for their help with the traditional discussions after the lectures and the Seehotel Waldstätterhof staff members. Collectively all made the 48th Bürgenstock a memorable experience. The 49th Bürgenstock will be held from the 4–9th of May 2014 and Antonio Echavarren will have the challenging task of matching the high quality of preceding conferences. As tradition suggests, the 2014 conference will certainly be as fascinating and stimulating and participation is highly recommended.
G.J.L.B. is a Royal Society University Research Fellow at the Department of Chemistry, University of Cambridge and a Principal Investigador FCT at the Instituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa. G.J.L.B. thanks the organising committee for the opportunity be part of the Bürgenstock Stereochemistry conference and for a JSP Fellowship in 2011.
Notes and references
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS.
- J. A. Hubbell and A. Chilkoti, Science, 2012, 337, 303–305 CrossRef.
- M. M. Martino, F. Tortelli, M. Mochizuki, S. Traub, D. Ben-David, G. A. Kuhn, R. Müller, E. Livne, S. A. Eming and J. A. Hubbell, Sci. Transl. Med., 2011, 3, 100ra189 CrossRef.
- C. Nembrini, A. Stano, K. Y. Dane, M. Ballester, A. J. van der Vlies, B. J. Marsland, M. A. Swartz and J. A. Hubbell, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, E989–E997 CrossRef CAS.
- T. H. Nguyen, S.-H. Kim, C. G. Decker, D. Y. Wong, J. A. Loo and H. D. Maynard, Nat. Chem., 2013, 5, 221–227 CrossRef CAS.
- R. J. Mancini, J. Lee and H. D. Maynard, J. Am. Chem. Soc., 2012, 134, 8474–8479 CrossRef CAS.
- D. P. Curran, M. K. Sinha, K. Zhang, J. J. Sabatini and D.-H. Cho, Nat. Chem., 2012, 4, 124–129 CrossRef CAS.
- X. Gao, S. K. Woo and M. J. Krische, J. Am. Chem. Soc., 2013, 135, 4223–4226 CrossRef CAS.
- Y. Lu, S. K. Woo and M. J. Krische, J. Am. Chem. Soc., 2011, 133, 13876–13879 CrossRef CAS.
- J. R. Zbieg, E. Yamaguchi, E. L. McInturff and M. J. Krische, Science, 2012, 336, 324–327 CrossRef CAS.
- M. Tredwell and V. Gouverneur, Angew. Chem., Int. Ed., 2012, 51, 11426–11437 CrossRef CAS.
- Z. Gao, Y. H. Lim, M. Tredwell, L. Li, S. Verhoog, M. Hopkinson, W. Kaluza, T. L. Collier, J. Passchier, M. Huiban and V. Gouverneur, Angew. Chem., Int. Ed., 2012, 51, 6733–6737 CrossRef CAS.
- G. L. Fiore, S. J. Rowan and C. Weder, Chem. Soc. Rev., 2013, 42, 7278–7288, 10.1039/c3cs35471g.
- M. V. Biyani, E. J. Foster and C. Weder, ACS Macro Lett., 2013, 2, 236–240 CrossRef CAS.
- G. M. Farinola and R. Ragni, Chem. Soc. Rev., 2011, 40, 3467–3482 RSC.
- H. Wang, G. Li, K. M. Engle, J.-Q. Yu and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6774–6777 CrossRef CAS.
- H. Wang, D. M. Guptill, A. Varela-Alvarez, D. G. Musaev and H. M. L. Davies, Chem. Sci., 2013, 4, 2844–2850 RSC.
- K. Gorska and N. Winssinger, Angew. Chem., Int. Ed., 2013, 52, 6820–6843 CrossRef CAS.
- J. P. Daguer, M. Ciobanu, S. Alvarez, S. Barluenga and N. Winssinger, Chem. Sci., 2011, 2, 625–632 RSC.
- M. Ciobanu, K.-T. Huang, J.-P. Daguer, S. Barluenga, O. Chaloin, E. Schaeffer, C. G. Mueller, D. A. Mitchell and N. Winssinger, Chem. Commun., 2011, 47, 9321–9323 RSC.
- B. Fierz and T. W. Muir, Nat. Chem. Biol., 2012, 417–427 CrossRef CAS.
- P. W. Lewis, M. M. Müller, M. S. Koletsky, F. Cordero, S. Lin, L. A. Banaszynski, B. A. Garcia, T. W. Muir, O. J. Becher and C. D. Allis, Science, 2013, 340, 857–861 CrossRef CAS.
- N. Winssinger, Artif. DNA: PNA XNA, 2012, 3, 105–108 CrossRef.
- I. Schoen, B. Pruitt and V. Vogel, Annu. Rev. Mater. Res., 2013, 43, 589–618, DOI:10.1146/annurev-matsci-062910-100407.
- M. Chabria, S. Hertig, M. L. Smith and V. Vogel, Nat. Commun., 2010, 1, 135 CrossRef.
- J.-i. Yoshida, H. Kim and A. Nagaki, ChemSusChem, 2011, 4, 331–340 CrossRef CAS.
- K. Saito, K. Ueoka, K. Matsumoto, S. Suga, T. Nokami and J.-i. Yoshida, Angew. Chem., Int. Ed., 2011, 50, 5153–5156 CrossRef CAS.
- T. Morofuji, A. Shimizu and J.-i. Yoshida, J. Am. Chem. Soc., 2013, 135, 5000–5003 CrossRef CAS.
- B. Cormary, F. Dumestre, N. Liakakos, K. Soulantica and B. Chaudret, Dalton Trans., 2013 10.1039/c3dt50870f.
| This journal is © The Royal Society of Chemistry 2013 |
