Supramolecular chains of high nuclearity {MnIII25} barrel-like single molecule magnets†
Dimosthenis P.
Giannopoulos
a,
Annaliese
Thuijs
b,
Wolfgang
Wernsdorfer
c,
Melanie
Pilkington
a,
George
Christou
b and
Theocharis C.
Stamatatos
*a
aDepartment of Chemistry, Brock University, St. Catharines, Ontario L2S 3A1, Canada. E-mail: tstamatatos@brocku.ca; Tel: +1-905-688-5550 Ext. 3400
bDepartment of Chemistry, University of Florida, Gainesville, Florida 32611-7200, USA
cInstitut Néel, CNRS, Nanoscience Department, BP 166, 380412 Grenoble Cedex 9, France
First published on 31st October 2013
Abstract
The first application of 1-methyl-1H-pyrrole-2-carbaldehyde oxime as a ligand for the coordination of paramagnetic transition metal ions has afforded a new {MnIII25} barrel-like cluster linked via Na+ cations into a 1D polymeric topology that exhibits single-molecule magnetic behaviour.
The field of single-molecule magnets (SMMs) has grown considerably over the past two decades to include families of transition metal complexes incorporating paramagnetic ions with moderate-to-high oxidation states,1 as well as complexes assembled from trivalent lanthanide2 and actinide3 metal ions. Research efforts in this field have been largely driven by the two requirements for molecules to exhibit SMM behaviour namely, a bistable electronic ground state arising from an often large, ground state spin value (S), coupled with magnetic anisotropy, attributed to a negative zero-field splitting (zfs) parameter (D).4 Such a combination affords an energy barrier (|D|S2) for reversal of the magnetization. Thus, at sufficiently low temperatures, these molecules function as nanoscale magnetic particles and straddle the classical/quantum interface by displaying not just classical magnetization hysteresis, but also quantum tunneling of magnetization (QTM) and quantum phase interference.5
In this context, SMMs have been proposed as promising candidates for potential applications in information storage and spintronics at the molecular level and for use as quantum bits in quantum computation.6 For SMMs to be incorporated into devices the molecules must be coupled to each other, and strategies to weakly couple SMMs while preserving their bistability are being actively pursued.7 Furthermore, in order for the magnetic properties of SMMs to be commercially viable, improvements in both their blocking temperatures and coercive fields are necessary. Strategies that enable the rational modification of the bridging units between SMMs represent a promising approach for maximizing these parameters.8,9 The self-assembly of SMMs into multi-dimensional architectures therefore remains a challenge for coordination chemists working at the interface of supramolecular chemistry and molecular magnetism.10
With respect to the preparation of novel families of SMMs, we and others have had success in developing high nuclearity 3d metal clusters based on 2-pyridyloxime ligands, (py)C(R)NOH (R = various; Scheme 1, left).7,11 More recently we have shown that the related 2-pyrrolyloximes (Scheme 1, middle) also act as bridging ligands for the coordination of paramagnetic metal centers. The simplest ligand initially chosen was pyrrole-2-carboxaldehyde oxime (praoH2; R′ = R′′ = H; Scheme 1, middle) which forms a family of Fe3, Fe6, Fe10 and Fe12 clusters, all with very small or zero S values.12 However, attempts to generate similar MnIII complexes, which may offer large magnetic anisotropy, were thwarted by the presence of the pyrrole N–H functionality, a well-known reducing group. In order to inhibit such redox activity, we have turned our attention to the closely related ligand, 1-methyl-1H-pyrrole-2-carbaldehyde oxime (mpraoH; R′ = Me, R′′ = H; Scheme 1, middle). Here we show that the alkylated mpraoH ligand is robust with respect to redox activity and supports the formation of high nuclearity clusters. The title compound behaves as an SMM, and the diamagnetic Na+ coordinated ions help organize the individual quantum spins on the SMMs into supramolecular chains.
Reaction of mpraoH with Mn(ClO4)2·6H2O, NEt3 and NaN3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in MeCN–MeOH (2
1 molar ratio in MeCN–MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) in air afforded a dark brown solution indicative of the presence of MnIII and/or MnIV ions. Layering this solution with Et2O afforded, after four days, X-ray quality, dark brown plate-like crystals of the 1D coordination polymer [Mn25NaO20(OMe)12(N3)12(mprao)12(H2O)2]n·8nMeOH (1·8MeOH)n in 30% yield.‡
1 v/v) in air afforded a dark brown solution indicative of the presence of MnIII and/or MnIV ions. Layering this solution with Et2O afforded, after four days, X-ray quality, dark brown plate-like crystals of the 1D coordination polymer [Mn25NaO20(OMe)12(N3)12(mprao)12(H2O)2]n·8nMeOH (1·8MeOH)n in 30% yield.‡
Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with the molecule possessing crystallographic Ci symmetry. The core of the cluster is held together by twelve μ4-O2−, eight μ3-O2−, six μ3- and six μ-OMe− ions, as well as the deprotonated oximato arms of twelve η1
with the molecule possessing crystallographic Ci symmetry. The core of the cluster is held together by twelve μ4-O2−, eight μ3-O2−, six μ3- and six μ-OMe− ions, as well as the deprotonated oximato arms of twelve η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η2
η2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μ3 mprao− groups (Fig. 1, top). The Mn25 core can be dissected into five parallel layers of three types with an ABCBA arrangement (Fig. 1, bottom). Layer A is a MnIII3 triangular unit with a capping μ3-O2− ion; layer B is a MnIII6 triangle that can be described as three corner-fused MnIII3 triangular moieties each capped by a μ3-O2− ion; and layer C is a disk-like MnIII7 unit, reminiscent of the known Anderson-type structure. Each layer is held together and linked to its neighbours by a combination of oxido, methoxido, and/or oximato bridges. Whilst the core geometry is similar to that previously observed for the mixed-valence MnII6MnIII18MnIV barrels [Mn25O18(OH)2(N3)12(pdm)6(pdmH)6]2+ and [Mn25O18(OH)(OMe)(hmp)6(pdm)6(pdmH)6]8+ (pdmH2 = 2,6-pyridinedimethanol; hmpH = 2-(hydroxymethyl)pyridine),13 the oxidation states of the Mn ions in 1 are all assigned to MnIII. The oxidation states were determined qualitatively by inspection of metric parameters and detection of MnIII Jahn–Teller (JT) elongation axes for all crystallographically independent Mn ions, as well as quantitatively by bond valence sum (BVS)14 calculations (Table S1, ESI†). In the case of the central MnIII atom (Mn6), which lies on a crystallographic inversion centre, both the intermediate for a MnII/MnIII ion BVS value and the absence of a clear JT axis are assigned to the static disorder among the three symmetry-equivalent O(15,19,22)–Mn(6)–O(15′,19′,22′) axes.15
μ3 mprao− groups (Fig. 1, top). The Mn25 core can be dissected into five parallel layers of three types with an ABCBA arrangement (Fig. 1, bottom). Layer A is a MnIII3 triangular unit with a capping μ3-O2− ion; layer B is a MnIII6 triangle that can be described as three corner-fused MnIII3 triangular moieties each capped by a μ3-O2− ion; and layer C is a disk-like MnIII7 unit, reminiscent of the known Anderson-type structure. Each layer is held together and linked to its neighbours by a combination of oxido, methoxido, and/or oximato bridges. Whilst the core geometry is similar to that previously observed for the mixed-valence MnII6MnIII18MnIV barrels [Mn25O18(OH)2(N3)12(pdm)6(pdmH)6]2+ and [Mn25O18(OH)(OMe)(hmp)6(pdm)6(pdmH)6]8+ (pdmH2 = 2,6-pyridinedimethanol; hmpH = 2-(hydroxymethyl)pyridine),13 the oxidation states of the Mn ions in 1 are all assigned to MnIII. The oxidation states were determined qualitatively by inspection of metric parameters and detection of MnIII Jahn–Teller (JT) elongation axes for all crystallographically independent Mn ions, as well as quantitatively by bond valence sum (BVS)14 calculations (Table S1, ESI†). In the case of the central MnIII atom (Mn6), which lies on a crystallographic inversion centre, both the intermediate for a MnII/MnIII ion BVS value and the absence of a clear JT axis are assigned to the static disorder among the three symmetry-equivalent O(15,19,22)–Mn(6)–O(15′,19′,22′) axes.15
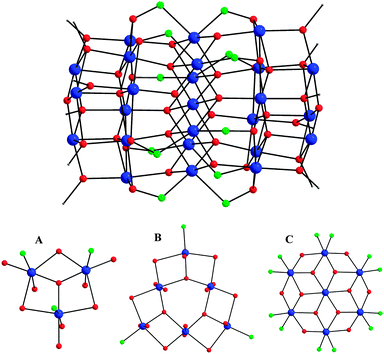 | ||
| Fig. 1 (top) Core structure of the {MnIII25} barrel. (bottom) ORTEP representations of the three types of constituent Mnx layers in 1. Colour scheme: MnIII blue; O red; N green; C grey. | ||
Peripheral ligation about the Mn25 core is provided by twelve terminally bound N3− ions, four of which assemble end-to-end linking the Mn25 units to a Na+ ion at each end, affording a 1D linear chain (Fig. 2). The coordination sphere of the octahedral Na+ cations is completed by two axially bonded H2O molecules. The shortest Mn⋯Mn separation between the Mn25 magnetic units is appreciably large (11.23(5) Å), while there are no other significant intermolecular interactions between the 1D chains. The overall barrel shape and nanometer size of the Mn25 unit in 1 are emphasized in the space-filling plots shown in Fig. S1 and S2 (ESI†); the cluster has a length of ∼18.5 Å and a diameter of ∼17.2 Å, excluding the H-atoms.
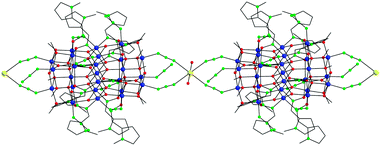 | ||
| Fig. 2 A small portion of the 1D polymeric structure of 1. Colour scheme as in Fig. 1; Na yellow. H-atoms are omitted for clarity. | ||
Solid-state, dc magnetic susceptibility (χM) data for an air-dried sample of 1 were collected in the temperature range 5–300 K and in an applied field of 0.1 T. The data reveal that χMT steadily decreases from 73.48 cm3 K mol−1 at 300 K to 20.62 cm3 K mol−1 at 5.0 K (Fig. S3, ESI†). The 300 K value is slightly less than the spin-only (g = 2) value of 75 cm3 K mol−1 for 25 MnIII non-interacting ions, indicating the presence of dominant antiferromagnetic exchange interactions within the cage. The 5 K value suggests that 1 possesses a fairly large ground state spin value of possibly S = 6; the spin-only (g = 2) value for S = 6 is 21 cm3 K mol−1. Given the size of the Mn25 molecule, and the resulting number of inequivalent exchange constants, it is not possible to determine the individual pairwise Mn2 exchange interaction parameters. Thus, we concentrated instead on characterizing the ground state spin, S, and the zfs parameter, D, by performing magnetization (M) vs. dc field measurements in a magnetic field and temperature range 1–70 kG and 1.8–10.0 K, respectively. Unfortunately, we were unable to obtain an acceptable fit for the data collected over the whole field range. This is a common problem in many large Mn clusters due to the population of low-lying excited states even at ultra-low temperatures, especially if some have an S value greater than that of the ground state.
A powerful complement to dc studies for determining the ground state of a system, and also to study magnetization dynamics, is ac magnetic susceptibility measurements which preclude any complications arising due to the presence of a dc field. These were performed in a 3.5 G ac field oscillating at different frequencies. The in-phase susceptibility (χΜ′) is shown as χΜ′T vs. T in Fig. S4 (ESI†) (top), and reveals several pertinent features: (i) χΜ′T decreases linearly with decreasing temperature in the 4–15 K range, indicating depopulation of a high density of excited states with spin S greater than that of the ground state, which is in agreement with the conclusion of the dc studies; (ii) extrapolation of the χΜ′T data from above ∼4.0 K to 0 K gives a value of ∼21 cm3 K mol−1, which is indicative of an S = 6 ground state with g = 2; (iii) below ∼4 K, there is a frequency-dependent decrease in χΜ′T and a concomitant appearance of frequency-dependent χΜ′′ signals (Fig. S4, bottom, ESI†); such signals are an indication of the superparamagnetic-like slow relaxation of an SMM.
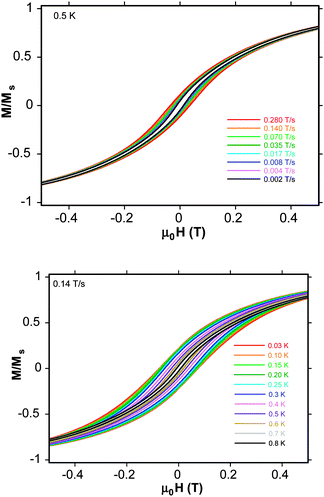
To confirm this last observation, magnetization vs. applied dc field data were collected on a single-crystal of 1·8MeOH down at 0.03 K on a micro-SQUID. Hysteresis loops are seen below ∼0.8 K whose coercivities increase with increasing sweep rate (Fig. 3, top) and with decreasing temperature (Fig. 3, bottom), confirming 1 to be an SMM. These loops do not show the steps characteristic of quantum tunnelling, as expected for large SMMs which are more susceptible to various step-broadening effects.10,13,16
It should be noted that complex 1 is simply too complicated to rationalize the S = 6 ground state on the basis of its structure alone. There are extensive spin frustration effects operating within the many fused Mn3 triangular units, which render any spin assignment inaccurate and superficial.
In conclusion, we have established that the N-alkylation of a 2-pyrrolyloxime stabilizes the molecule with respect to oxidation. The resultant mprao− ligand is therefore able to bridge MnIII centres affording a large polynuclear barrel-shaped {MnIII25} cluster that is structurally related to the examples of mixed-valence Mn25 barrel-like SMMs reported in the literature.13 To date, this system represents the highest nuclearity Mn cluster which is organized into a 1D polymer through chelation with diamagnetic metal centers.17 Given the apparent stability of these barrel-like clusters in multiple oxidation states, the redox activity of 1 is being actively pursued. Furthermore, strategies aimed at the selective replacement of the diamagnetic Na+ cations with paramagnetic 4f-metal ions, targeting the rational assembly of 1 into new classes of SCMs, are currently under investigation.
This work was supported by Brock University and NSERC Discovery Grant (Th.C.S and M.P), CRC and CFI (M.P), the National Science Foundation (DMR-1213030 to G.C), the ERC Advanced Grant MolNanoSpin No. 226558 (WW).
Notes and references
- G. Aromí and E. K. Brechin, Struct. Bonding, 2006, 122, 1 CrossRef.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed.
- J. D. Rinehart and J. R. Long, J. Am. Chem. Soc., 2009, 131, 12558 CrossRef CAS PubMed.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed.
- W. Wernsdorfer and R. Sessoli, Science, 1999, 284, 133 CrossRef CAS PubMed.
- A. Candini, S. Klyatskaya, M. Ruben, W. Wernsdorfer and M. Affronte, Nano Lett., 2011, 11, 2634 CrossRef CAS PubMed.
- T. N. Nguyen, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2011, 133, 20688 CrossRef CAS PubMed.
- W.-X. Zhang, R. Ishikawa, B. Breedlove and M. Yamashita, RSC Adv., 2013, 3, 3772 RSC.
- D. Liu, Q. Zhou, Y. Chen, F. Yang, Y. Yu, Z. Shi and S. Feng, Cryst. Growth Des., 2010, 10, 2661 CAS and references therein.
- (a) E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2006, 45, 7722 CrossRef CAS PubMed; (b) E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Inorg. Chem., 2009, 48, 5049 CrossRef CAS PubMed and references therein.
- (a) C. J. Milios, T. C. Stamatatos and S. P. Perlepes, Polyhedron, 2006, 25, 134 CrossRef CAS; (b) C. Dendrinou-Samara, C. M. Zaleski, A. Evagorou, J. W. Kampf, V. L. Pecoraro and D. P. Kessissoglou, Chem. Commun., 2003, 2668 RSC.
- (a) A. B. Canaj, M. Siczek, A. P. Douvalis, T. Bakas, T. Lis and C. J. Milios, Polyhedron, 2013, 52, 1411 CrossRef CAS; (b) E. S. Koumousi, A. Routzomani, T. N. Nguyen, D. P. Giannopoulos, C. P. Raptopoulou, V. Psycharis, G. Christou and T. C. Stamatatos, Inorg. Chem., 2013, 52, 1176 CrossRef CAS PubMed.
- (a) M. Murugesu, M. Habrych, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2004, 126, 4766 CrossRef CAS PubMed; (b) T. C. Stamatatos, K. A. Abboud, W. Wernsdorfer and G. Christou, Angew. Chem., Int. Ed., 2007, 46, 884 CrossRef CAS PubMed.
- W. Liu and H. H. Thorp, Inorg. Chem., 1993, 32, 4102 CrossRef CAS.
- (a) E. S. Koumousi, M. J. Manos, C. Lampropoulos, A. J. Tasiopoulos, W. Wernsdorfer, G. Christou and T. C. Stamatatos, Inorg. Chem., 2010, 49, 3077 CrossRef CAS PubMed; (b) J. P. Fackler and A. Avdeef, Inorg. Chem., 1984, 13, 1864 CrossRef.
- T. C. Stamatatos, A. Vinslava, K. A. Abboud and G. Christou, Chem. Commun., 2009, 2839 RSC.
- S. Nayak, Y. Lan, R. Clérac, C. E. Anson and A. K. Powell, Chem. Commun., 2008, 5698 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystallographic data (CIF format), synthetic details, and various structural and magnetism figures of 1. CCDC 950209. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc47094f |
‡ Air-dried solid analyzed (C, H, N) as 1. Calcd (found): C, 24.57 (24.68); H, 3.04 (3.33); N, 20.46 (20.34%). Crystal data for 1: C92H156Mn25NaN60O54, Mw = 4363.25, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a = 16.233(3) Å, b = 16.707(3) Å, c = 17.691(3) Å, α = 63.270(6), β = 80.447(7), γ = 65.254(6), V = 3890.2(12) Å3, T = 150(2) K, Z = 1, Dc = 1.862 g cm−3, 54 with a = 16.233(3) Å, b = 16.707(3) Å, c = 17.691(3) Å, α = 63.270(6), β = 80.447(7), γ = 65.254(6), V = 3890.2(12) Å3, T = 150(2) K, Z = 1, Dc = 1.862 g cm−3, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391 reflections collected, 13 391 reflections collected, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 unique (Rint = 0.0659), R1 [I > 2σ(I)] = 0.0917, wR2 = 0.2206 (F2, all data). 608 unique (Rint = 0.0659), R1 [I > 2σ(I)] = 0.0917, wR2 = 0.2206 (F2, all data). |
| This journal is © The Royal Society of Chemistry 2014 |