 Open Access Article
Open Access ArticleZn(II) chloride-catalyzed direct coupling of various alkynes with acetals: facile and inexpensive access to functionalized propargyl ethers†
Itaru
Suzuki
,
Makoto
Yasuda
* and
Akio
Baba
*
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871, Japan. E-mail: yasuda@chem.eng.osaka-u.ac.jp; baba@chem.eng.osaka-u.ac.jp; Fax: +81-6-6879-7387; Tel: +81-6-6879-7386
First published on 27th September 2013
Abstract
The coupling of acetals with various alkynes was achieved using only 1 mol% of inexpensive and mild Lewis acid ZnCl2, which furnished propargyl ethers. The coupling was catalyzed by Zn(OMe)Cl, which was generated in situ to form an alkynylzinc species. This protocol was allowed to expand to a one-pot subsequent reaction with allylchlorosilane to obtain a 1,4-enyne product.
Alkynylation is a fundamental and valuable method1 for the preparation of bioactive compounds2 and charge transport materials.3 The employment of alkynyl metal agents such as alkynyllithiums,4 -silanes,5 -stannanes,6 and -boranes7 makes for versatile methods, but these cannot avoid the annoying preparation of, and the incompatibility that results from, various functional groups. To overcome these issues, the direct use of terminal alkynes has been the focus from an environmental point of view.8
Our group has reported the direct synthesis of alkynylstannanes from various terminal alkynes and Bu3SnOMe as catalyzed by ZnBr2, in which Zn(OMe)Br is generated by transmetalation between Bu3SnOMe and ZnBr2 and plays a key role in producing an active alkynylzinc species in situ (Scheme 1a).9 We expected the reaction between dimethyl acetals and ZnBr2 to generate oxonium cations along with Zn(OMe)Br,10 which may be an alternative formation of Zn(OMe)Br. This idea prompted us to develop the reaction between terminal alkynes and acetals in the presence of ZnBr2 wherein the generated alkynyl zinc from Zn(OMe)Br was expected to promote the coupling (Scheme 1b). Some examples of coupling between acetals and alkynes have been recently reported, but these generated a cation of metals like Au+ for the activation of alkynes11 or more than one equimolar amount of base for alkynyl metal generation.12 Fortunately, direct coupling could be promoted by using only a catalytic amount of inexpensive ZnBr2 or ZnCl2 to furnish propargyl ethers, and it was a surprise that a weak Lewis acid such as ZnCl2 worked with no additives.
 | ||
| Scheme 1 Comparison of previous work with this work. | ||
An investigation into the reaction conditions was commenced. Benzaldehyde dimethyl acetal (1a) did not react with 1-decyne (2a) without a catalyst under toluene refluxing conditions (Table 1, entry 1). The addition of 10 mol% of ZnBr2 provided the coupling product 3aa in an 81% yield (entry 2). A higher yield was realized when ZnCl2 was utilized (entry 3). The reaction was completed in 12 h using only 1 mol% loading of ZnCl2–Et2O, furnishing 3aa quantitatively (entry 4). ZnI2 and Zn(OTf)2 gave moderate yields, while Zn(OAc)2 showed no effect (entries 5–7). Employment of mild Lewis acids like InCl3, CuCl2, and BiCl3 gave moderate yields (entries 8–10). In contrast, strong Lewis acids such as AlCl3, TiCl4, BF3·OEt2, or SnCl4 did not promote the reaction at all (entries 11–14). The combined Lewis acid, which was reported as an effective catalyst for the coupling using alkynylsilanes,13 gave no product (entry 15).
|
|
||
|---|---|---|
| Entry | Catalyst | Yieldb (%) |
| a Reaction conditions: 1a (2.0 mmol), 2a (1.0 mmol), and a catalyst (0.10 mmol) were refluxed in toluene (1 mL) for 24 h. b 1H NMR yield. The value in parentheses indicates the isolated yield. c Catalyst (0.01 mmol), 12 h. | ||
| 1 | None | 0 |
| 2 | ZnBr2 | 81 |
| 3 | ZnCl2 | 90 |
| 4c | ZnCl2–Et2O | 99 (86) |
| 5 | ZnI2 | 76 |
| 6 | Zn(OTf)2 | 50 |
| 7 | Zn(OAc)2·2H2O | 0 |
| 8 | InCl3 | 55 |
| 9 | CuCl2 | 65 |
| 10 | BiCl3 | 40 |
| 11 | AlCI3 | 0 |
| 12 | TiCl4 | 0 |
| 13 | BF3·OEt2 | 5 |
| 14 | SnCl4 | 0 |
| 15 | SnCl4 + ZnCl2 | 0 |
The optimized reaction conditions (Table 1, entry 4) were applicable to the series of terminal alkynes listed in Table 2. Aryl alkynes 2b–d also afforded the corresponding adducts 3ab–3ad, but the electron-rich alkyne 2d was not as effective owing to a low pKa of the terminal proton. Ester and silicon moieties did not disturb the reaction (entries 4 and 5). Alkynes 2g and 2h bearing active propargyl positions were also effectively coupled with an acetal (entries 6 and 7). Chloro and cyano groups were intact after the reaction (entries 8 and 9). Cyclohexylacetylene (2k) gave the desired product 3ak in a high yield. It is noteworthy that a variety of alkynes, including functionalized alkyls, were applicable in contrast to previous methods that were limited to aromatic alkynes.11,12 The mildness of our method could be the reason for the wide application.
|
|
||||
|---|---|---|---|---|
| Entry | Alkyne | Product | Yieldb (%) | |
| a Reaction conditions: 1a (2.0 mmol), alkyne (1.0 mmol), and a catalyst (0.01 mmol) were refluxed in toluene (1 mL) for 12 h. b 1H NMR yield. The values in parentheses indicate isolated yields. c Catalyst (0.03 mmol). d Catalyst (0.05 mmol). | ||||
| 1c |

|
2b | 3ab | 93 (86) |
| 2d | 2c | 3ac | 69 (43) | |
| 3 | 2d | 3ad | 99 (98) | |
| 4c |

|
2e | 3ae | 91 (80) |
| 5c |

|
2f | 3af | 97 (86) |
| 6 |
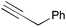
|
2g | 3ag | 94 (94) |
| 7c |

|
2h | 3ah | 99 (77) |
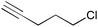
| 8 |
|
2i | 3ai | 99 (78) |
| 9c |

|
2j | 3aj | 99 (82) |
| 10 |
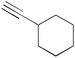
|
2k | 3ak | 91 (81) |
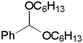
Next, the effect of acetals was investigated (Table 3). Diethyl acetal 1b gave a high yield upon increasing the amount of catalyst to 0.05 mmol (entry 1). However, no reaction took place when using dihexyl acetal 1c even with a 5 mol% loading of the catalyst (entry 2). An electron-withdrawing group on an aromatic ring in an acetal decreased the yield of 3 plausibly due to the destabilization of the oxonium cation intermediate (entry 5). Isochroman derivative 1g gave an excellent yield (entry 6). The alkynylation of cynnamyl aldehyde dimethyl acetal (1h) was also achieved (entry 7) to give the mixture of regioisomers 3ha, which also suggested that the reaction proceeded via an oxonium cation species. Unfortunately, no product was obtained from aliphatic acetal 1i (entry 8).
|
|
||||
|---|---|---|---|---|
| Entry | Acetal | Product | Yieldb (%) | |
| a Reaction conditions: 1 (2.0 mmol), 2a (1.0 mmol), and catalyst (0.01 mmol) were refluxed in toluene (1 mL) for 12 h. b 1H NMR yield. Values in parentheses indicate isolated yields. c Catalyst (0.05 mmol). d Catalyst (0.03 mmol). | ||||
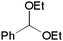
| 1c |
|
1b | 3ba | 91 (77) |
| 2 |
|
1c | 0 | |
| 3 |

|
1d | 3da | 92 (89) |
| 4d | 1e | 3ea | 99 (85) | |
| 5c | 1f | 3fa | 99 (90) | |
| 6 |

|
1g | 3ga | 99 (85) |
| 7c |

|
1h | 3ha | 99 (78) |
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 66 Z = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
||||
| 8 |

|
1i | 0 | |
To explain the results in entries 1 and 2 in Table 3, the effect of an alcohol that was generated in situ, plausibly as a by-product, was investigated. No reaction proceeded in a sealed vessel (Scheme 2). Moreover, the addition of 0.2 mL of methanol decreased the yield to 29%. These results indicate the importance of removing the produced alcohol from the reaction media, because the alcohol would hamper the interaction between an acetal and ZnCl2.
 | ||
| Scheme 2 Disturbing effect of an alcohol by-product. | ||
To confirm the incorporation of an alkynylzinc species, which was proposed in our previous report,9 the alkynylzinc prepared using alkynylbromide 4 and zinc metal was treated with acetal 1a.14 To our delight, the desired coupling product was obtained in a 59% yield (Scheme 3). This result strongly indicates that the reaction contained an alkynyzinc species.
 | ||
| Scheme 3 Reaction of alkynylzinc 5 with acetal 1a. | ||
We investigated whether this protocol would allow the alkynylation of aldehydes, because the catalytic alkynylation of aldehydes with terminal alkynes has been reported.15 The fact that there was no reaction of alkyne 2c with benzaldehyde (6) (Scheme 4) implies that the active species, Zn(OMe)Cl, generated from dimethyl acetals is essential for the catalytic coupling reaction.
 | ||
| Scheme 4 Reaction of aldehyde 6 with alkyne 2c. | ||
A plausible reaction mechanism is shown in Scheme 5. ZnCl2 activates the acetal to give zinc species 7, which interacts with an alkyne and leads to the formation of alkynylzinc 8. The alkynylzinc 8 reacts with acetal 1via an oxonium cation 9 and a zincate complex to afford the desired product 3 along with the regeneration of 7. The kinetic study of the coupling was carried out by GC (see ESI†) and showed that the reaction was dependent on the first order of each component (v = k[1a][2a][catalyst], k; 4.06 × 10−2 mol−2 L2 s−1, T = 130 °C). The result and implication of containing an alkynylzinc as shown in Scheme 3 might indicate that the interaction between an acetal and alkynylzinc 8 is the rate-limiting step.
 | ||
| Scheme 5 Plausible reaction mechanism. | ||
The produced propargyl ether 3aa was found to subsequently react with allylchlorosilane 10 in a one-pot treatment, where the allylation was completed in 30 min at room temperature, yielding 1,5-enyne 11 (Scheme 6). The isolated 3aa did not react with 10 in the absence of ZnCl2, which apparently suggested the catalytic role of ZnCl2 in the substitution of the OMe moiety to the allyl one.
 | ||
| Scheme 6 One-pot allylation of the product 3aa. | ||
In conclusion, we developed an alkynylation of acetals with various alkynes including alkyls that can be catalyzed by inexpensive ZnCl2. This reaction needs no expensive metal catalyst, such as gold,11 nor does it need additives.12 The product, propargyl ether, was functionalized without isolation, which shows that this reaction is clean enough to effectively undergo further transformation.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (Japan) and the Shorai Foundation for Science and Technology.
Notes and references
- For recent reviews on alkynylation of carbonyl compounds, see: (a) S. Guillarme, K. Plé, A. Banchet, A. Liard and A. Haudrechy, Chem. Rev., 2006, 106, 2355 CrossRef CAS PubMed; (b) B. M. Trast and A. H. Weiss, Adv. Synth. Catal., 2009, 351, 963 CrossRef . For a transition metal catalyzed cross coupling, see: ; (c) K. Sonogashira, J. Organomet. Chem., 2002, 653, 46 CrossRef CAS; (d) E.-i. Negishi and L. Anastasia, Chem. Rev., 2003, 103, 1979 CrossRef CAS PubMed; (e) H. Doucet and J.-C. Hierso, Angew. Chem., Int. Ed., 2007, 46, 834 CrossRef CAS PubMed . For a review on electrophilic alkynylation, see: ; (f) J. P. Brand and J. Waser, Chem. Soc. Rev., 2012, 41, 4165 RSC.
- (a) K. C. Nicolau and W.-M. Dai, Angew. Chem., 1991, 103, 1453 ( Angew. Chem., Int. Ed. Engl. , 1991 , 30 , 1387 ) CrossRef; (b) M. E. Maier, Synlett, 1995, 13 CrossRef CAS PubMed; (c) A. L. K. Shi Shu and R. R. Tykwinski, Angew. Chem., Int. Ed., 2006, 45, 1034 CrossRef PubMed; (d) R. E. Minto and B. J. Blacklock, Prog. Lipid Res., 2008, 47, 233 CrossRef CAS PubMed; (e) B. W. Gung, C. R. Chim., 2009, 12, 489 CrossRef CAS PubMed.
- (a) A. Harriman and R. Ziessel, Chem. Commun., 1996, 1707 RSC; (b) N. J. Long and C. K. Williams, Angew. Chem., Int. Ed., 2003, 42, 2586 CrossRef CAS PubMed; (c) K. A. Green, M. P. Cifuentes, M. Samoc and M. G. Humphrey, Coord. Chem. Rev., 2011, 255, 2530 CrossRef CAS PubMed; (d) K. A. Green, M. P. Cifuentes, M. Samoc and M. G. Humphrey, Coord. Chem. Rev., 2011, 255, 2025 CrossRef CAS PubMed; (e) G. Bottari, D. D. Díaz and T. Torres, J. Porphyrins Phthalocyanines, 2006, 10, 1083 CrossRef CAS; (f) F. Silvestri and A. Marrocchi, Int. J. Mol. Sci., 2010, 11, 1471 CrossRef CAS PubMed.
- G. Wu and M. Huang, Chem. Rev., 2006, 106, 2596 CrossRef CAS PubMed.
- S. E. Denmark and M. H. Ober, Aldrichimica Acta, 2003, 36, 75 CAS.
- S. Pascual and A. M. Echavarren, in Tin Chemistry, ed. A. G. Davies, John Wiley & Sons Ltd., Chichester, 2008, pp. 579–606 Search PubMed.
- J. Jiao and Y. Nishihara, J. Organomet. Chem., 2012, 721–722, 3 CrossRef CAS PubMed.
- (a) N. K. Anand and E. M. Carreira, J. Am. Chem. Soc., 2001, 123, 9687 CrossRef CAS; (b) R. Takita, Y. Fukuta, R. Tsuji, T. Ohshima and M. Shibasaki, Org. Lett., 2005, 7, 1363 CrossRef CAS PubMed; (c) R. Takita, K. Yakura, T. Ohshima and M. Shibasaki, J. Am. Chem. Soc., 2005, 127, 13760 CrossRef CAS PubMed.
- K. Kiyokawa, N. Tachikake, M. Yasuda and A. Baba, Angew. Chem., Int. Ed., 2011, 50, 10393 CrossRef CAS PubMed.
- ZnBr2 catalyzed reaction of acetals with acetyl chloride was reported. See: M. A. Berliner and K. Belecki, J. Org. Chem., 2005, 70, 9618 CrossRef CAS PubMed.
- (a) C. Li, F. Mo, W. Li and J. Wang, Tetrahedron Lett., 2009, 50, 6053 CrossRef CAS PubMed; (b) M. Zhang, Y. Wang, Y. Yang and X. Hu, Adv. Synth. Catal., 2012, 354, 981 CrossRef CAS.
- P. Maity, H. D. Srinivas and M. P. Watson, J. Am. Chem. Soc., 2011, 133, 17142 CrossRef CAS PubMed.
- M. Hayashi, A. Inubushi and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1988, 61, 4037 CrossRef CAS.
- G. M. R. Tombo, E. Didier and B. Loubinoux, Synlett, 1990, 547 CrossRef.
- (a) J.-i. Ito, R. Asai and H. Nishiyama, Org. Lett., 2010, 12, 3860 CrossRef CAS PubMed; (b) P. K. Dhondi and J. D. Chisholm, Org. Lett., 2006, 8, 67 CrossRef CAS PubMed; (c) Y. Asano, K. Hara, H. Ito and M. Sawamura, Org. Lett., 2007, 9, 3901 CrossRef CAS PubMed; (d) T. Ishikawa, T. Mizuta, K. Hagiwara, T. Aikawa, T. Kudo and S. Saito, J. Org. Chem., 2001, 68, 3702 CrossRef PubMed; (e) M. Yamashita, K.-i. Yamada and K. Tomioka, Adv. Synth. Catal., 2005, 347, 1649 CrossRef CAS; (f) T. Weil and P. R. Schreiner, Eur. J. Org. Chem., 2005, 2213 CrossRef CAS , also see; ref. 8.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc46570e |
| This journal is © The Royal Society of Chemistry 2013 |



