 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced imine synthesis in water: from surfactant-mediated catalysis to host–guest mechanisms†
Kamel
Meguellati
a,
Ali
Fallah-Araghi
a,
Jean-Christophe
Baret
b,
Abdeslam
El Harrak
c,
Thomas
Mangeat
c,
Carlos M.
Marques
d,
Andrew D.
Griffiths
*a and
Sylvain
Ladame
*ae
aInstitut de Science et d'Ingénierie Supramoléculaires (ISIS), Université de Strasbourg, CNRS UMR 7006, 8 allée Gaspard Monge, F-67083 Strasbourg Cedex, France. E-mail: griffiths@unistra.fr
bMax-Planck Institute for Dynamics and Self-organization, Am Fassberg 17, D-37077 Göttingen, Germany
cRainDance Technologies France, 8 allée Gaspard Monge, F-67083 Strasbourg Cedex, France
dInstitut Charles Sadron, CNRS UPR 22, 23 rue du Loess, 67034 Strasbourg Cedex 2, France
eDepartment of Bioengineering, Imperial College London, London, SW7 2AZ, UK. E-mail: sladame@imperial.ac.uk
First published on 10th October 2013
Abstract
An environment-responsive and fluorogenic reaction is reported and used as a model system to demonstrate experimentally three mechanisms of enhanced imine synthesis in water using either surfactants (below and above their CMC) or double-stranded DNA (acting as a reaction host).
In solution, entropic factors thermodynamically disfavour the formation of larger molecules from smaller ones. The enthalpy of the reaction (ΔH) depends mainly on the change in bond energy and solvation energy. The entropy of the reaction (ΔS) is a measure of the change in possibility of movement of the constituents of the reaction: the change in the degrees of freedom of overall translational movement and rotational movement is most significant whereas the contribution by vibrations and internal rotations is small.1 The formation of one product molecule from two reactant molecules (2 → 1 reaction) in solution will result in a high loss of mobility (and hence a loss of entropy) equivalent to 6 degrees of freedom (3 translational and 3 rotational). Compartmentalisation (e.g. in receptors or capsules) is one of the mechanisms that have been invoked to overcome this entropic problem.2 In confined geometries, chemical reactions take place under crowded conditions that can affect the availability of reaction partners (on the reaction time scale) and by this means will influence the outcome of the reaction. Herein, we demonstrate experimentally that synthetic reactions of imine formation, otherwise highly unfavourable in water, can be enhanced through (1) surfactant-mediated catalysis, (2) confinement within micelles or (3) a host–guest mechanism, using double-stranded DNA as a host.
As a model system to study these three mechanisms we used the reversible reaction of a non-fluorescent amine 1 with a very weakly fluorescent aldehyde 2 to form a fluorescent imine 3 in water (Fig. 1). Fluorescent imine 3 differs from the well-known trimethine cyanine dyes by one C → N substitution in the polymethine chain, thus making its formation reversible and thermodynamically controlled.3 Because of the intrinsic fluorogenic properties of this reaction, we used fluorescence spectroscopy to monitor changes in reaction efficiency (apparent equilibrium constant Keq, first and second order rate constants k−1 and k1) in response to various environmental stimuli.
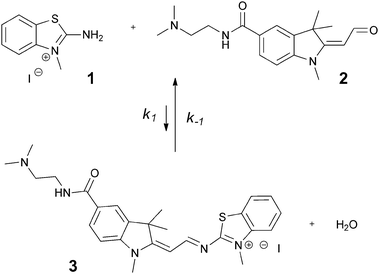 | ||
| Fig. 1 Reversible synthesis of fluorescent imine 3 from non-fluorescent amine 1 and weakly fluorescent aldehyde 2. | ||
Water soluble Fischer's base aldehyde 2 was synthesised in 4 steps from commercially available 5-hydrazinobenzoic acid (see ESI†). N-Methyl-2-amino-benzothiazolium salt 1 was synthesised in one step from 2-aminobenzothiazole.3
The formation of imine 3 from amine 1 and aldehyde 2 was first investigated in bulk solvent and its photophysical properties determined by fluorescence spectroscopy. In aqueous solution, formation of imine 3 was barely detectable at stoichiometric reactant concentrations ≤0.5 mM, but when working at a ≥5 mM concentration of both amine and aldehyde, formation of imine 3 was easily detectable (ESI†). Nevertheless, even under these conditions, imine 3 formation was both slow (k1 = 1.84 × 10−5 M−1 s−1) and thermodynamically unfavourable (Keq = 2.71 × 10−2 M−1).
It is well known that the rate and yield of many chemical reactions can be enhanced in micellar systems.4 In some cases, micelles function as catalysts since they possess catalytically active groups and/or the transition state of the reaction is stabilised (reduction of the activation energy) by interaction with the polar head groups of the surfactant. In other cases the enhancement is thought to be due to the micellar microenvironment (activity coefficient effects). However, for bimolecular reactions, the dominant effect in many cases is thought to be due to reactants being concentrated relative to the surrounding water phase through interaction with the micelle surface or insertion into the micelle itself (entropy effects).4 The imine formation reaction was therefore carried out in the presence of various self-assembled systems like surfactants (cationic, anionic and non-ionic) or polyelectrolytes (Table 1). Various concentrations, above and below the critical micelle concentration (CMC), were examined to identify the effect of the monomer assembly on reaction efficiency.
| Surfactant or polymer | Charge | CMCb (%w/w) at 25 °C | 0.03% w/w surfactant | 0.8% w/w surfactant | ||
|---|---|---|---|---|---|---|
| K eq (M−1) | k 1 (M−1 s−1) | K eq (M−1) | k 1 (M−1 s−1) | |||
| a Reactions were carried out with stoichiometric quantities of amine 1 and aldehyde 2 (15 mM each) and in the presence of 0.03% or 0.8% w/w surfactant or polymer and monitored by fluorescence spectroscopy (λem = 520 nm). The surfactants and polymers tested were sodium dodecylsulfate (SDS), Triton X-100, cetyl trimethylammonium bromide (CTAB), Pluronic F-108, Pluronic F-127, Polyvinylpyrrolidone (PVP), Zonyl and Polyacrylic acids (PAA250 and PAA805). b CMC values are from the literature; N/A, data not available. c Zonyl was provided by DuPont under Zonyl™. (See Fig. S3, ESI for raw data). | ||||||
| SDS | Anionic | 0.23 | 0.054 | 4.00 × 10−5 | 1.520 | 51.5 × 10−5 |
| PAA250 | Anionic | N/A | 0.028 | 1.26 × 10−5 | 0.350 | 14.9 × 10−5 |
| PAA805 | Anionic | N/A | 0.031 | 1.75 × 10−5 | 0.140 | 4.92 × 10−5 |
| CTAB | Cationic | 0.033 | 0.027 | 1.35 × 10−5 | 0.048 | 1.26 × 10−5 |
| Triton X-100 | Non-ionic | 0.012 | 0.026 | 0.98 × 10−5 | 0.055 | 1.66 × 10−5 |
| F-108 | Non-ionic | 3.88 | 0.026 | 1.59 × 10−5 | 0.041 | 1.64 × 10−5 |
| F-127 | Non-ionic | 0.567 | 0.025 | 1.42 × 10−5 | 0.043 | 1.93 × 10−5 |
| PVP | Non-ionic | N/A | 0.027 | 1.70 × 10−5 | 0.045 | 0.98 × 10−5 |
| Zonylc | Non-ionic | N/A | 0.027 | 1.44 × 10−5 | 0.065 | 1.27 × 10−5 |
Only the anionic molecules (SDS, PAA250 and PAA805) proved capable of substantially enhancing the apparent k1 and the apparent Keq of the reaction of imine formation. In contrast, the addition of cationic (CTAB) and non-ionic (Pluronic F-127, PVP, Triton X-100, Zonyl and Synperonic F-108) molecules resulted in little or no change in imine production, as expected considering the cationic nature of both reactants and of the final imine 3.
The enhancement with anionic surfactants was more marked when micelles are present in the solution (i.e. above the CMC). At 0.03%, all of the surfactants tested are below the CMC, and no supramolecular auto-assembly of the surfactant was possible. However, at 0.8%, with the exception of PAA250, PAA805 and Pluronic F-108, all the surfactants are above the CMC and were shown to form micellar structures, the size of which was determined by dynamic light scattering (DLS, see ESI†). We then decided to focus on the case of SDS, varying systematically the SDS concentration from 0.15–3.0 × CMC (CMC = 9 mM) in order to study the effect of micellar self-assembly. Below the CMC, the apparent Keq increased only slowly with concentration, while a dramatic shift of the position of equilibrium was observed above the CMC. In contrast, the apparent k1 increased linearly with SDS concentration below the CMC, and increased only slowly (if at all) above (Fig. 2). The free energy changes5 are summarised in Fig. 3 for [SDS] = 0; 1 × CMC and 3 × CMC (see Table S1, ESI†).
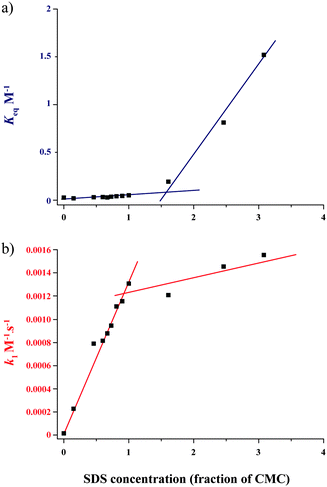 | ||
| Fig. 2 Kinetics and thermodynamics of imine 3 synthesis in the presence of different concentrations of SDS. A stoichiometric mixture of amine 1 and aldehyde 2 (15 mM each) was reacted in the presence of various SDS concentrations and monitored by fluorescence spectroscopy (λem = 520 nm). (a) Plot of the equilibrium constants (Keq) calculated from the concentration of imine 3 at equilibrium (t = 3 h). (b) Plot of the second order rate constants (k1) calculated from the initial rate of formation of imine 3. SDS concentration is expressed as a molar fraction of CMC. Experimental data were fitted by linear regression (coloured lines). | ||
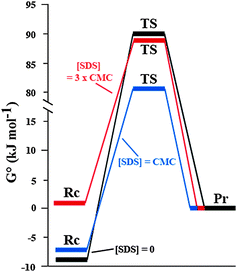 | ||
| Fig. 3 Free energy diagrams of the imine formation reaction in bulk. G0 of the reactants, Rc (amine 1 and aldehyde 2), the transition state, TS, and the product, Pr (imine 3) are shown with G0 of the product defined as zero. The reaction in bulk, with no SDS (black line), with SDS concentration equal to the CMC (9 mM; blue line) and SDS concentration 3 times the CMC (27 mM; red line). | ||
It can be seen that equal to or below the CMC, the predominant effect is due to reduction in the G0TS of the transition state, probably as a result of favourable electrostatic interactions between the anionic surfactant and the transition state (Fig. 3). However, below the CMC, SDS only subtly affects the position of equilibrium, likely due to hydrophobic interactions between the SDS tails (in the absence of micelle formation) inducing a local increase in reactant concentration which results in the small observed increase in G0Rc of the reactants. Thus SDS in solution is functioning as a true catalyst of the reaction. Above the CMC, the G0TS with and without SDS are almost identical, indicating that transition state stabilization does not play an important role. However, the equilibrium position of the reaction is substantially shifted towards the formation of imine, probably largely due to the increase in local concentration of reactants due to sequestration in the micelles, which results in a large increase in G0Rc of the reactants relative to the transition state and product.
Considering the capacity of trimethine cyanine dyes to bind into the minor groove of B-DNA6 and based on the principles of dynamic covalent chemistry (DCC)7 we reasoned that the reversible fluorogenic synthesis of imino trimethine cyanine dye 3 could be triggered in the presence of double-stranded DNA.
Experiments were carried out using stoichiometric concentrations of amine 1 and aldehyde 2 (500 μM each in water pH7) in the absence and in the presence of varying concentrations of calf-thymus double-stranded DNA (6–60 μM in base pairs8) and the reaction was monitored by fluorescence spectroscopy over 24 hours. As shown in Fig. 4, whilst no imine was detectable in the absence of DNA at this concentration of reactants, reaction efficiency was significantly enhanced in the presence of DNA concentrations as low as 6 μM. Similar experiments were then carried out but in the presence of various nucleic acid templates: (i) a stoichiometric mixture of the four deoxyribonucleotide triphosphate (dNTPs); (ii) a mixture of uncharged deoxyribonucleotides and (iii) a solution of single-stranded 12-mer DNA oligonucleotide (ssDNA). Unlike B-DNA, none of these proved capable of efficiently enhancing the imine 3 formation reaction (see ESI†). These results are consistent with the hypothesis that imine 3 is stabilised only upon binding to double-stranded DNA (likely via binding into the minor groove like the equivalent trimethine cyanine dye).6 Hence only dsDNA can act as a host to drive the equilibrium of this (otherwise highly unfavourable) reaction toward the formation of the fluorescent imino dye. Although there exist a few examples of reversible reactions that use nucleic acids as hosts for the selection of specific ligands,9 the current approach for the detection of dsDNA offers the advantage of easy and highly sensitive monitoring by fluorescence spectroscopy.
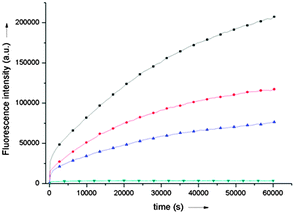 | ||
| Fig. 4 Kinetics of formation of imine 3 in bulk solvent (water, pH7) at 25 °C from a stoichiometric mixture of amine 1 and aldehyde 2 (500 μM each) in the presence of increasing concentrations of calf thymus double-stranded DNA: 60 μM (black), 15 μM (red), 10 μM (blue) and 6 μM (green). Monitored by fluorescence spectroscopy (λem = 520 nm). | ||
The dynamic nature of the reaction of imine formation can be exploited to drive the equilibrium either forward or backwards. Since the reaction involves the loss of a molecule of water, imine synthesis is very unfavourable in water. Herein, we used a fluorogenic reaction of imine formation as a model system to study experimentally the responsive behaviour of imines to external stimuli including free surfactants, surfactant micelles and double-stranded DNA. Anionic surfactants (e.g. SDS) below the CMC act as true catalysts by stabilising the reaction transition state. At concentrations above the CMC, they can shift the equilibrium position toward the imine product, likely via increased local concentration within micellar compartments. Addition of double-stranded DNA also resulted in an enhancement of reaction efficiency, likely due to a stabilisation of the reaction product (fluorescent imine 3) within the DNA minor groove. This environment-responsive and fluorogenic system may therefore have applications as a DNA hybridisation probe, enabling the hybridisation of two complementary DNA strands to be detected by monitoring the ‘templated’ formation of a fluorescent dye within the B-DNA host (whilst no fluorescence is observed in the presence of single-stranded DNA).
Notes and references
- M. Page and W. Jencks, Proc. Natl. Acad. Sci. U. S. A., 1971, 68, 1678 CrossRef CAS.
- F. Hof and J. Rebek Jr, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4775 CrossRef CAS PubMed.
- K. Meguellati, M. Spichty and S. Ladame, Org. Lett., 2009, 11, 1123 CrossRef CAS PubMed.
- E. H. Cordes, Pure Appl. Chem., 1978, 50, 617 CrossRef CAS; T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., Int. Ed., 2005, 44, 7174 CrossRef PubMed.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1935, 31, 875 RSC; H. Eyring, J. Chem. Phys., 1935, 3, 107 CrossRef CAS.
- K. C. Hannah, R. R. Gil and B. A. Armitage, Biochemistry, 2005, 44, 15924 CrossRef CAS PubMed; H. J. Karlsson, M. Eriksson, E. Perzon, B. Akerman, P. Lincoln and G. Westman, Nucleic Acids Res., 2003, 31, 6227 CrossRef PubMed.
- J. Li, P. Nowak and S. Otto, J. Am. Chem. Soc., 2013, 135, 9222 CrossRef CAS PubMed; S. Ladame, Org. Biomol. Chem., 2008, 6, 219 Search PubMed.
- DNA concentrations are expressed in base pairs and were calculated using an absorption coefficient at 260 nm of 13
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1. See D. E. Graves and R. M. Wadkins, J. Biol. Chem., 1989, 264, 7262 CAS.
200 M−1 cm−1. See D. E. Graves and R. M. Wadkins, J. Biol. Chem., 1989, 264, 7262 CAS. - Z. Qing, X. He, D. He, K. Wang, F. Xu, T. Qing and X. Yang, Angew. Chem., Int. Ed., 2013, 52, 9719, DOI:10.1002/anie.201304631; P. C. Gareiss, K. Sobczak, B. R. McNaughton, P. B. Palde, C. A. Thornton and B. L. Miller, J. Am. Chem. Soc., 2008, 130, 16254 CrossRef PubMed; S. Ladame, A. M. Whitney and S. Balasubramanian, Angew. Chem., Int. Ed., 2005, 44, 5736 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and product characterisation. See DOI: 10.1039/c3cc46461j |
| This journal is © The Royal Society of Chemistry 2013 |
