 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancing optical absorption of metal–organic frameworks for improved visible light photocatalysis†
Maxim A. Nasalevich*a, Maarten G. Goestena, Tom J. Savenijeb, Freek Kapteijna and Jorge Gascon*a
aCatalysis Engineering, Department of Chemical Engineering, Delft University of Technology, Julianalaan 136, Delft, 2628 BL, The Netherlands. E-mail: M.Nasalevich@tudelft.nl; J.Gascon@tudelft.nl; Fax: +31 1527 85006; Tel: +31 1527 84851
bOptoelectronic Materials, Department of Chemical Engineering, Delft University of Technology, Julianalaan 136, Delft, 2628 BL, The Netherlands
First published on 23rd September 2013
Abstract
NH2-MIL-125(Ti) has been post-synthetically functionalized with dye-like molecular fragments. The new material (methyl red-MIL-125(Ti)) exhibits improved light absorption over a wide range of the visible spectrum, and shows enhanced photocatalytic oxidation activity under visible light illumination. The consequences of functionalization and the bottlenecks in MOF photochemistry are studied in detail.
Metal–organic frameworks are crystalline porous solids possessing high surface areas and highly tuneable properties. This relatively new class of coordination compounds is being considered for application in different fields, such as heterogeneous catalysis, gas storage/separation and imaging.1–4 Among the wide variety of properties of interest, several MOFs exhibit very interesting optoelectronic features. For instance, recent synthetic achievements have delivered chemically robust MOFs displaying photocatalytic activity. In particular Ti- and Zr-based frameworks were successfully employed for a variety of photocatalytic transformations such as CO2 reduction, H2 evolution and oxidation of organic compounds.5–7 The first metal–organic framework constructed using titanium clusters and terephthalates was reported by Férey and co-workers.8 However, most MOFs based on terephthalate linkers exhibit optical absorption only in the UV-region, while undoubtedly the main challenge in photocatalysis lies in the complete utilization of the solar spectrum. In order to tackle this issue, several authors have demonstrated that by inclusion of amine moieties (using amino-terephthalic acid as a linker), it is possible to slightly extend light absorption of MIL-125(Ti) to the visible region.9–11 In this work we demonstrate that post-synthetic modification (PSM)12 of NH2-MIL-125(Ti) with antenna-like moieties can further extend visible light absorption, improving the photocatalytic performance of the framework under solar light illumination.
MIL-125(Ti) and NH2-MIL-125 were synthesized according to the method described elsewhere.13 NH2-MIL-125 was post-synthetically functionalized following a diazotization reaction similar to that recently reported by Burrows and co-workers.14 At the first stage the amino groups of the framework reacted with nitrosonium ions, NO+, generated in situ by the HCl–NaNO2 pair (ESI†). The temperature of the reaction mixture was kept at 0 °C to avoid the denitrogenation of the diazonium salt. The second step is the reaction of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N+-MIL-125(Ti) with diethylaniline, yielding a material referred to as methyl red-MIL-125(Ti) (MR-MIL-125(Ti)), in which the functional group resembles the well-known organic dye. During the synthesis we observed the suspension colour changing from yellow to almost white in the first step and then to dark orange in the second step (see video in ESI†).
N+-MIL-125(Ti) with diethylaniline, yielding a material referred to as methyl red-MIL-125(Ti) (MR-MIL-125(Ti)), in which the functional group resembles the well-known organic dye. During the synthesis we observed the suspension colour changing from yellow to almost white in the first step and then to dark orange in the second step (see video in ESI†).
The XRD patterns of MIL-125(Ti) and as-synthesized NH2-MIL-125(Ti) correspond well to those reported previously and remain unchanged after the PSM (ESI†), demonstrating the non-destructive transformation of the framework. The three materials were subjected to N2-physisorption analysis. As expected, pore volume decreases upon functionalization. DRIFT analysis demonstrates the disappearance of the amino-stretchings of NH2-MIL-125(Ti) (3519.5 and 3388.4 cm−1) after the modification (ESI†). However the analysis of the fingerprint region did not provide a direct proof of the formation of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. In order to confirm the designed modification, MR-MIL-125(Ti) was digested in concentrated KOH and analysed by NMR spectroscopy. Fig. 1 displays the 1H NMR spectrum of the digested methyl red-MIL-125(Ti). Resonances in the alkyl region undoubtedly demonstrate the successful functionalization. Quantitative analysis (ESI†) suggests that 26% to 30% of the linkers were functionalised. The relatively low degree of functionalization is not surprising when considering the bulkiness of the MR moiety and the mechanism of the diazotization reaction, in which denitrogenation of the diazonium salt may take place. Because of these reasons, we speculate that the highest degree of functionalization will not be higher than 50%, since successful functionalization of two adjacent linkers will be very unlikely.
N bond. In order to confirm the designed modification, MR-MIL-125(Ti) was digested in concentrated KOH and analysed by NMR spectroscopy. Fig. 1 displays the 1H NMR spectrum of the digested methyl red-MIL-125(Ti). Resonances in the alkyl region undoubtedly demonstrate the successful functionalization. Quantitative analysis (ESI†) suggests that 26% to 30% of the linkers were functionalised. The relatively low degree of functionalization is not surprising when considering the bulkiness of the MR moiety and the mechanism of the diazotization reaction, in which denitrogenation of the diazonium salt may take place. Because of these reasons, we speculate that the highest degree of functionalization will not be higher than 50%, since successful functionalization of two adjacent linkers will be very unlikely.
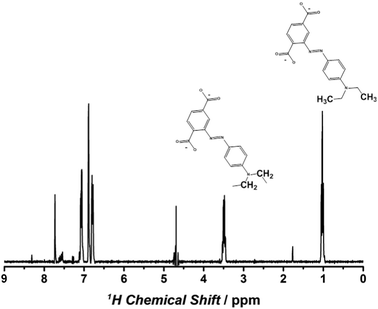 | ||
| Fig. 1 1H-NMR patterns of MR-MIL-125(Ti) digested in KOH. The alkyl signals confirm the designed modification. | ||
Optical absorption of the different materials was investigated using UV/Vis spectroscopy in diffuse reflectance mode. The main absorption bands of MIL-125(Ti) are located in the UV region. As expected, the presence of an electron donating substituent in the aromatic ring strongly affects the optical properties of the material. NH2-MIL-125(Ti) exhibits two absorption bands at 325 and 465 nm attributed to ATA2−Ti4+ → ATA−Ti3+ transition within the subunits of the framework.15–17 Once the material is modified, a clear red shift is observed with the absorption edge reaching almost 700 nm. This significant visible light absorption enhancement can be associated with a higher level of conjugation of the aromatic system in the linker. The longer wavelength transitions of the MOF suggest that most of the modified linkers exist in the trans-form, since the cis- and trans-isomers of the analogous methyl red molecule embedded in rigid polymers absorb at <400 and 565 nm respectively.18 We attribute the preference of the trans-isomer to a more efficient packing in the micropores of the framework. The calculated band gap energies were found to be 2.46 and 1.93 eV for NH2- and MR-MIL-125(Ti) respectively (ESI†). As a result of the functionalization, MR-MIL-125(Ti) absorbs 100% more light in the visible region than NH2-MIL-125(Ti) (ESI†).
The photocatalytic activity of MR-MIL-125(Ti) was assessed in the selective oxidation of benzyl alcohol to benzaldehyde. Oxidation of alcohols to corresponding aldehydes is one of the most important reactions in organic synthesis.19 The aldehydes can then be used for the production of fragrances and flavours.20 The reactions were carried out in CH3CN taking into account the recent reports of the importance of the solvent for aerobic organic oxidation on NH2-UiO-66 by Wang et al.21 In our experiments a home-built photocatalytic reactor was employed illuminated from the top by a 150 Xe fiber light source filtered by a 400 nm cut-on filter (ESI†) to ensure that only visible light is used.
The formation of benzaldehyde in the course of reaction is shown in Fig. 3. Blank experiments without illumination/catalyst did not yield any quantifiable amount of benzaldehyde after 24 hours of reaction. Both NH2-MIL-125 and MR-MIL-125 are active under visible light illumination. However, even on a weight basis the functionalized MOF shows a higher activity. The reaction rates of benzaldehyde formation for NH2-MIL-125(Ti) and MR-MIL-125(Ti) under the reaction conditions were found to be 77.6 and 86.7 nmol g−1 min−1 respectively (circa 40% improvement in the photocatalytic performance per atom of Ti present in the structure, see ESI†). Although the activities found in this work are rather moderate, they are well in line with the values reported for visible light carbon dioxide reduction and hydrogen evolution using NH2-MIL-125(Ti).9,15 Benzaldehyde was the only product detected in the liquid phase suggesting that the selectivity of the process approaches 100%. Additional experiments have shown that MR-MIL-125(Ti) can be easily recycled without any loss of activity (ESI†). This is a clear advantage over bulk TiO2, where deactivation has been observed in similar photo-oxidation reactions due to irreversible product adsorption.22 The improvement of the photocatalytic activity in the case of MR-MIL-125(Ti) with respect to the parent framework is associated with the enhanced optical absorption of the solid due to the extended conjugation of the aromatic system of the ligand (see Fig. 2).
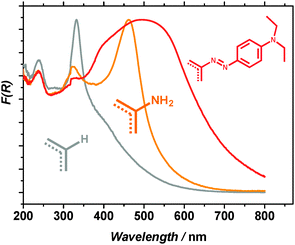 | ||
| Fig. 2 Diffuse reflectance spectra of the materials investigated in this study: MIL-125(Ti) (grey), NH2-MIL-125(Ti) (orange) and MR-MIL-125(Ti) (red). | ||
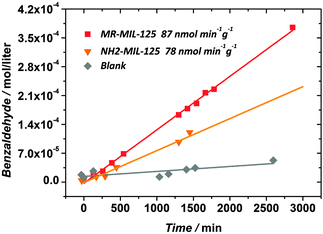 | ||
| Fig. 3 Photocatalytic benzaldehyde evolution. No catalyst (grey), NH2-MIL-125(Ti) (orange) and MR-MIL-125(Ti) (red). 200 μL of benzyl alcohol/12 mL CH3CN, 150 W Xe lamp. 12 mg of NH2-MIL-125(Ti) and 18 mg of MR-MIL-125(Ti). | ||
These results unambiguously demonstrate the possibility of tuning the optical absorption in MOFs, in line with our previous reports and with the most recent work by Walsh and co-workers.16,23 More importantly, we also demonstrate that improving light sensitivity only yields moderate gains in photocatalytic performance. In contrast to TiO2, the HOMO/valence band of the MIL-125 framework mainly consists of the π-orbitals of the aromatic ring of the linker.24 This explains the influence of ring substituents on the electronic excitation processes in the material. The difference between the 100% gain in light absorption and only 40% improvement in catalytic activity can be explained as follows: (i) optical absorption is not the unique factor contributing to the enhancement in activity, the difference in the adsorption properties should also be taken into account; (ii) not every photon within the absorption range possesses the necessary energy to drive the photocatalytic transformation. In order to gain more insight into these phenomena, we measured charge mobility in the parent framework by means of time-resolved microwave conductivity (TRMC). According to our results, MIL-125(Ti) shows a poor photoconductance (mobility ∼10−5 cm2 V−1 s−1 upon 340 nm illumination, ESI†). This conductance is significantly suppressed upon lowering the temperature to 153 K, in clear contrast to pure TiO2, with mobilities in the range of 1 cm2 V−1 s−1 nearly independent of temperature.25,26 This difference suggests thermally activated hopping as the main mechanism for the charge transport due to the isolation of the Ti clusters by the organic linkers in the MOF.27 Indeed, such clusters are too far apart to fulfil the Mott transition conditions, being approximately 4 Bohr radii.28,29 Moreover, in MIL-125(Ti), the distance between linkers is circa 7 Å, therefore, no efficient π–π stacking is possible30 and there is hardly any orbital overlap, keeping the electrons preferentially in a localized state. This fact demonstrates that the MIL-125(Ti) series (and MOFs in general) should be seen as an array of self-assembled molecular catalysts rather than as classical semiconductors. Indeed, the optical absorption spectra depicted in Fig. 2 should be considered as sets of individual discrete absorption bands, and the HOMO–LUMO gap terminology should be used in order to describe the discrete characteristics of the light-induced transitions in these coordination compounds. Therefore, the performance of MR-MIL-125(Ti) can be considered as contributions from two individual components: NH2-MIL-125(Ti) and MR-MIL-125(Ti). The ratio of the two as determined by the quantitative NMR analysis is 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Thus 30% of the linkers that are functionalized are responsible for the 40% gain in activity.
30. Thus 30% of the linkers that are functionalized are responsible for the 40% gain in activity.
In this communication, a straightforward PSM has been applied to improve the optical absorption of NH2-MIL-125(Ti). Post-synthetic functionalization of the organic linkers with the diazonium salt followed by the reaction with a nucleophilic species like diethylaniline yields the incorporation of dye-like moieties in the MOF scaffold. The resulting MR-MIL-125(Ti) displays a significant increase in light absorption as compared to the parent MOF. When applied in the selective photo-oxidation of benzyl alcohol under visible light illumination, the MR-MIL-125(Ti) framework displays an enhanced photocatalytic performance. This improvement is associated with the improved optical absorption of the catalyst.
In order to rationalize the catalytic results, photoconductance of MIL-125(Ti) has been explored using time-resolved microwave conductivity. Temperature-dependent signals of ∼10−5 cm2 V−1 s−1 were detected. This low conductivity clearly demonstrates that the MIL-125(Ti) series should be seen as an array of self-assembled molecular catalysts rather than as classical semiconductors.
We would like to gratefully acknowledge the Dutch National Research School Combination Catalysis Controlled by Chemical Design (NRSC-Catalysis) for funding this research. We thank Maria C. Fravventura for assistance with the TRMC experiments.
Notes and references
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957–968 CrossRef CAS PubMed.
- E. V. Ramos-Fernandez, C. Pieters, B. van der Linden, J. Juan-Alcañiz, P. Serra-Crespo, M. W. G. M. Verhoeven, H. Niemantsverdriet, J. Gascon and F. Kapteijn, J. Catal., 2012, 289, 42–52 CrossRef CAS PubMed.
- J. Juan-Alcaniz, J. Gascon and F. Kapteijn, J. Mater. Chem., 2012, 22, 10102–10118 RSC.
- S. Couck, E. Gobechiya, C. E. A. Kirschhock, P. Serra-Crespo, J. Juan-Alcañiz, A. Martinez Joaristi, E. Stavitski, J. Gascon, F. Kapteijn, G. V. Baron and J. F. M. Denayer, ChemSusChem, 2012, 5, 740–750 CrossRef CAS PubMed.
- J. L. Wang, C. Wang and W. Lin, ACS Catal., 2012, 2, 2630–2640 CrossRef CAS.
- C. G. Silva, I. Luz, F. X. Llabrés I Xamena, A. Corma and H. García, Chem.–Eur. J., 2010, 16, 11133–11138 CrossRef CAS PubMed.
- C. Wang, Z. Xie, K. E. Dekrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 13445–13454 CrossRef CAS PubMed.
- M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Férey, J. Am. Chem. Soc., 2009, 131, 10857–10859 CrossRef CAS PubMed.
- Y. Horiuchi, T. Toyao, M. Saito, K. Mochizuki, M. Iwata, H. Higashimura, M. Anpo and M. Matsuoka, J. Phys. Chem. C, 2012, 116, 20848–20853 CAS.
- M. De Miguel, F. Ragon, T. Devic, C. Serre, P. Horcajada and H. García, ChemPhysChem, 2012, 13, 3651–3654 CrossRef CAS PubMed.
- M. Kim and S. M. Cohen, CrystEngComm, 2012, 14, 4096–4104 RSC.
- S. M. Cohen, Chem. Rev., 2011, 112, 970–1000 CrossRef PubMed.
- M. A. Moreira, J. C. Santos, A. F. P. Ferreira, J. M. Loureiro, F. Ragon, P. Horcajada, P. G. Yot, C. Serre and A. E. Rodrigues, Microporous Mesoporous Mater., 2012, 158, 229–234 CrossRef CAS PubMed.
- D. Jiang, L. L. Keenan, A. D. Burrows and K. J. Edler, Chem. Commun., 2012, 48, 12053–12055 RSC.
- Y. Fu, D. Sun, Y. Chen, R. Huang, Z. Ding, X. Fu and Z. Li, Angew. Chem., Int. Ed., 2012, 51, 3364–3367 CrossRef CAS PubMed.
- J. Gascon, M. D. Hernández-Alonso, A. R. Almeida, G. P. van Klink, F. Kapteijn and G. Mul, ChemSusChem, 2008, 1, 981–983 CrossRef CAS PubMed.
- H. Khajavi, J. Gascon, J. M. Schins, L. D. A. Siebbeles and F. Kapteijn, J. Phys. Chem. C, 2011, 115, 12487–12493 CAS.
- G. J. Lee, D. Kim and M. Lee, Appl. Opt., 1995, 34, 138–143 CrossRef CAS PubMed.
- S. V. Ley and A. Madin, in Comprehensive Organic Synthesis, ed. M. T. Barry and F. Ian, Pergamon, Oxford, 1991, pp. 251–289 Search PubMed.
- S. Higashimoto, N. Kitao, N. Yoshida, T. Sakura, M. Azuma, H. Ohue and Y. Sakata, J. Catal., 2009, 266, 279–285 CrossRef CAS PubMed.
- J. Long, S. Wang, Z. Ding, S. Wang, Y. Zhou, L. Huang and X. Wang, Chem. Commun., 2012, 48, 11656–11658 RSC.
- A. R. Almeida, M. Calatayud, F. Tielens, J. A. Moulijn and G. Mul, J. Phys. Chem. C, 2011, 115, 14164–14172 CAS.
- C. H. Hendon, D. Tiana, M. Fontecave, C. Sanchez, L. D'arras, C. Sassoye, L. Rozes, C. Mellot-Draznieks and A. Walsh, J. Am. Chem. Soc., 2013, 135, 10942–10945, DOI:10.1021/ja405350u.
- A. Walsh and C. R. A. Catlow, ChemPhysChem, 2010, 11, 2341–2344 CrossRef CAS PubMed.
- M. C. Fravventura, D. Deligiannis, J. M. Schins, L. D. A. Siebbeles and T. J. Savenije, J. Phys. Chem. C, 2013, 117, 8032–8040 CAS.
- T. J. Savenije, A. Huijser, M. J. W. Vermeulen and R. Katoh, Chem. Phys. Lett., 2008, 461, 93–96 CrossRef CAS PubMed.
- R. A. Marcus, J. Chem. Phys., 1956, 24, 966–978 CrossRef CAS.
- Heterogeneous photocatalysis, ed. M. Schiavello, Wiley, Chichester, UK, 1997 Search PubMed.
- N. F. Mott, Can. J. Phys., 1956, 34, 1356–1368 CrossRef CAS PubMed.
- V. Barone, M. Casarin, D. Forrer, M. Pavone, M. Sambi and A. Vittadini, J. Comput. Chem., 2009, 30, 934–939 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the synthesis, photocatalytic tests, X-ray diffraction data, N2 adsorption isotherms, DRIFTs, NMR and TRMC traces. See DOI: 10.1039/c3cc46398b |
| This journal is © The Royal Society of Chemistry 2013 |
