Open Access Article
Open Access ArticleI2–PPh3 mediated spiroannulation of unsaturated β-dicarbonyl compounds. The first synthesis of (±)-negundoin A†
Rubén Tapiaa, Ma José Canoa, Hanane Bouanoua, Esteban Alvareza, Ramón Alvarez-Manzanedab, Rachid Chahboun*a and Enrique Alvarez-Manzaneda*a
aDepartamento de Química Orgánica, Facultad de Ciencias, Instituto de Biotecnología, Universidad de Granada, 18071 Granada, Spain. E-mail: eamr@ugr.es; rachid@ugr.es; Fax: +34 958 24 80 89; Tel: +34 958 24 80 89
bDepartamento de Química Orgánica, Facultad de Ciencias, Universidad de Almería, 04120 Almería, Spain
First published on 9th September 2013
Abstract
An efficient and stereoselective spiroannulation of unsaturated enols is reported. Unsaturated β-dicarbonyl compounds undergo cyclization by reaction with catalytic I2–PPh3, affording the corresponding spiro enol ether derivatives, with complete regio- and stereoselectivity, under mild conditions. Utilizing this new methodology, the first total synthesis of the anti-inflammatory diterpene negundoin A and a naturally occurring trypanocidal aldehyde is reported.
Spirocompounds, with two rings joined at a single atom, are widely found in Nature. Among these, some spiroethers present particular interest due to their important biological properties. Spirodihydrobenzofuran derivatives, such as the complement inhibitor K-76,1 the antagonist of endothelin and an inhibitor of HIV-1 protease stachybotrylactam,2 or cytotoxic stypoldione (1),3 are some representative bioactive spiroethers. A series of nor-diterpenes, bearing a characteristic tricyclic structure containing a spiro enol ether group with an α,β-unsaturated aldehyde, acid or ester, have recently been isolated from different species of the genus Vitex, which are widely used in folk medicine in some Asian countries. Representative examples are negundoin C (2), a potent anti-inflammatory aldehyde isolated from V. negundo, acid 3 (negundoin B) and ester 4 (negundoin A),4 together with the trypanocidal aldehydes 5 and 6, found in V. trifolia5 (Fig. 1).
 | ||
| Fig. 1 Some bioactive natural spiro ethers. | ||
Only a few syntheses have been reported for some of these spirodihydrobenzofuran derivatives, i.e. K-76 and stachybotrylactam; in all cases, the key step is the spiroannulation of the suitable drimane (bicyclic sesquiterpene) phenol, under acidic conditions.1b,c,6 To date, no syntheses for spiro enol ether derivatives, such as compounds 2–6, have been reported.
Spiro tetrahydrofurylidene systems similar to that presented by negundoin A (4) have been previously elaborated by reaction of an oxirane with the dianion of a β-ketoester and the subsequent treatment with acid.7,8 The construction of the spiro enol ether framework of compounds 2–6 could be achieved by spiro annulation of the enol derived from the corresponding unsaturated β-dicarbonyl compound 7 under suitable reaction conditions (Scheme 1). Alternatively, O-alkylation can take place affording the corresponding pyran derivative. Cyclization of β-dicarbonyl compound type 7 can also occur through the C-alkylation of the corresponding enol, leading to type 8 compounds. In some cases other C-cyclization processes can take place after the attack of an olefinic or an allyl carbon on the carbonyl group, affording the corresponding β-hydroxy carbonyl compound.9 In most of the reactions described under acidic conditions processes involving C-cyclization have been reported.10–12 Some examples of obtention of tetrahydrofurylidene derivatives by cyclization of unsaturated β-ketoesters catalyzed using SnCl413 or Pd(II)14 have been described. Under these conditions, alkyl 6-methyl-3-oxo-6-heptenoates afford the corresponding spiro compound resulting from the favourable enol O-attack on the most substituted olefinic carbon. The stereoselectivity of these processes remains uncertain.13a,14 However, the spiroannulation of unsaturated β-dicarbonyl compounds bearing a tetrasubstituted olefinic bond, such as compound 7, to achieve compounds 2–6 involves certain difficulty due to the variety of possible alternative cyclizations discussed above. In order to search cyclization conditions favouring the required O-alkylation process, the behaviour of unsaturated β-ketoesters 9, 10, 12 and 13 under different cyclization conditions, including acidic ones, was studied. In most cases the cyclization process is not stereoselective, leading to a mixture of compounds, resulting from a C-alkylation reaction.15
 | ||
| Scheme 1 Alternative cyclization processes for unsaturated β-dicarbonyl compounds. | ||
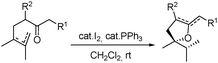
In the course of our investigations into the use of the I2–PPh3 system16 we found that unsaturated β-ketoesters are efficiently transformed, in the presence of catalytic amounts of this reagent, into the corresponding spiro enol ethers. Thus, the β-ketoester 9 was converted with complete regio- and stereoselectivity into the spiro compound 21 by treatment with this system, in dichloromethane, at room temperature for 8 h (see Table 1). Ketoester 10 gave the same results under the above reaction conditions. Similarly, compound 11 was transformed in good yield into the spiro compound 22. In order to optimize the reaction conditions and establish the scope of this reaction, some other β-ketoesters, β-ketoaldehydes and β-diketones were then studied. In all cases the corresponding spiro enol ethers were obtained with complete regio- and stereoselectivity. β-Ketoaldehydes (entries 7 and 8) show a similar behaviour. It should be noted that aldehyde 25 was the only spirane derivative obtained as a mixture of E–Z stereoisomers (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); the reason for this behaviour remains unclear. Compound 16 was transformed under the same reaction conditions into the spirane 5, a trypanocidal aldehyde isolated from V. trifolia.5 The optical rotation of synthetic aldehyde 5 ([α]D +1.2; c 8.6, CHCl3) and the spectroscopic properties were similar to those reported for the natural product. β-Diketones (entries 9 and 10) also produced the corresponding spiro enol ether derivatives. The relative stereochemistry of all the above spiro compounds was established on the basis of nOe experiments. On the other hand, β-ketoester 19 and β-diketone 20, containing a prenyl substituent, afforded under the above conditions the corresponding enol ether bearing a dihydropyran ring. In order to rule out the participation of hydriodic acid in this I2–PPh3 mediated process, the behaviour of β-ketoester 9 against this acid reagent was investigated. Compound 9 remained unaltered after treating with 55% aq. HI in dichloromethane at room temperature for 48 h. Under reflux, decomposition was observed.
1); the reason for this behaviour remains unclear. Compound 16 was transformed under the same reaction conditions into the spirane 5, a trypanocidal aldehyde isolated from V. trifolia.5 The optical rotation of synthetic aldehyde 5 ([α]D +1.2; c 8.6, CHCl3) and the spectroscopic properties were similar to those reported for the natural product. β-Diketones (entries 9 and 10) also produced the corresponding spiro enol ether derivatives. The relative stereochemistry of all the above spiro compounds was established on the basis of nOe experiments. On the other hand, β-ketoester 19 and β-diketone 20, containing a prenyl substituent, afforded under the above conditions the corresponding enol ether bearing a dihydropyran ring. In order to rule out the participation of hydriodic acid in this I2–PPh3 mediated process, the behaviour of β-ketoester 9 against this acid reagent was investigated. Compound 9 remained unaltered after treating with 55% aq. HI in dichloromethane at room temperature for 48 h. Under reflux, decomposition was observed.
| |||
|---|---|---|---|
| Entry | β-Dicarbonyl compound | t (h) | Product |
| 1 |  | 8 |  |
| 2 | 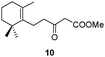 | 8 |  |
| 3 |  | 5 |  |
| 4 |  | 5 |  |
| 5 |  | 5 |  |
| 6 |  | 5 |  |
| 7 | 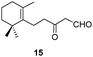 | 5 |  |
| 8 |  | 5 |  |
| 9 |  | 3 | 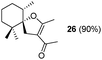 |
| 10 |  | 5 | 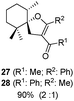 |
| 11 | 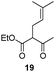 | 5 | 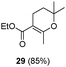 |
| 12 |  | 5 | 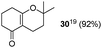 |
A first fact to be considered in rationalizing these results is the complete anti stereoselectivity of the addition process. When the I2–PPh3 system is utilized, an anti concerted process, precluding the formation of an intermediate carbocation, must take place. A possible mechanism, consistent with the experimental results, is postulated. The spirocyclization process is depicted in Scheme 2. Under the reaction conditions, the trisubstituted or exocyclic carbon–carbon double bond (compounds 9 and 13; 14 and 16) undergoes isomerization to the most stable tetrasubstituted derivatives. The enol hydroxyl group acts as a nucleophile and a proton donor simultaneously. The OH group, activated by the phosphonium ion +PPh3I, transfers the proton by the β side of the olefinic bond of the adjacent molecule. The latter undergoes the simultaneous intramolecular nucleophilic O-attack on carbon 1, affording intermediate I, which is a precursor of the spirane compound (Scheme 2). The complete regioselectivity of the cyclization process could be attributed to the preference for the transference of protons on the less hindered carbon 2. The preference for the β side proton transference could be attributed to the α side steric hindrance due to the keto ester moiety. As expected, compounds 19 and 20 afforded the dihydropyran ethers 29 and 30, respectively, resulting from the OH attack on the most substituted olefinic carbon.
 | ||
| Scheme 2 A possible mechanism for the I2–PPh3 mediated cyclization of β-dicarbonyl compounds. | ||
Utilizing the above spirocyclization of β-ketoesters, negundoin A (4) was synthesized (Scheme 3), utilizing the key intermediate hydroxyketone 32, a terpenoid found in Copaiba oil,20 which is easily prepared by the titanocene-catalyzed cyclization of (2E,5E)-9,10-epoxy-farnesyl acetone ketal (31).21 The spectroscopic properties of synthetic negundoin A (4) were identical to those reported for the natural product.4
 | ||
| Scheme 3 Synthesis of negundoin A (4). | ||
The authors thank the Spanish Ministry of Science and Innovation (Project CTQ2009-09932) and the Regional Government of Andalucia (Project P11-CTS-7651 and assistance for the FQM-348 group) for financial support.
Notes and references
- (a) H. Kaise, M. Shinohara, W. Miyazaki, T. Izawa, Y. Nakano, M. Sugawara, K. Sugiura and K. Sasaki, J. Chem. Soc., Chem. Commun., 1979, 726 RSC; (b) E. J. Corey and J. Das, J. Am. Chem. Soc., 1982, 104, 5551 CrossRef CAS; (c) J. E. McMurry and M. D. Erion, J. Am. Chem. Soc., 1985, 107, 2712 CrossRef CAS . For a recent review concerning K-76 and structurally related compounds see: ; (d) E. L. Larghi and T. S. Kaufman, ARKIVOC, 2011,(Pt. vii), 49 CAS.
- (a) B. E. Roggo, F. Petersen, M. Sills, J. L. Roesel, T. Moerker and H. H. Peter, J. Antibiot., 1996, 49, 13 CrossRef CAS PubMed; (b) B. E. Roggo, P. Hug, S. Moss, A. Stampfli, H. P. Kriemler and H. H. Peter, J. Antibiot., 1996, 49, 374 CrossRef CAS PubMed.
- (a) D. Faulkner, Nat. Prod. Rep., 2002, 19, 1 CAS and references therein; (b) O. M. M. Sabry, S. Andrews, K. L. McPhail, D. E. Goeger, A. Yokochi, K. T. Lepage, T. F. Murray and W. H. Gerwick, J. Nat. Prod., 2005, 68, 1022 CrossRef CAS PubMed.
- C.-J. Zheng, B.-K. Huang, Y. Wang, Q. Ye, T. Han and Q.-Y. Zhang, Bioorg. Med. Chem., 2010, 18, 175 CrossRef CAS PubMed.
- (a) F. Kinchi, K. Matsuo, M. Ito, T.-K. Qui and G. Honda, Chem. Pharm. Bull., 2004, 52, 1492 CrossRef; (b) C.-J. Zheng, J. Pu, H. Zhang, T. Han, K. Rahman and L.-P. Qin, Fitoterapia, 2012, 83, 49 CrossRef CAS PubMed.
- (a) A. S. Kende, W.-P. Deng, M. Zhongand and X.-C. Guo, Org. Lett., 2003, 5, 1785 CrossRef CAS PubMed; (b) W.-P. Deng, M. Zhong, X.-C. Guo and A. S. Kende, J. Org. Chem., 2003, 68, 7422 CrossRef CAS PubMed.
- M. Yamaguchi and I. Hirao, Chem. Lett., 1985, 337 CrossRef CAS.
- F. Marc, B. Soulet, D. Serramedan and B. Delmond, Tetrahedron, 1994, 50, 3381 CrossRef CAS.
- As an example see compounds 39, 41 and 42 in the ESI†.
- (a) S. Escher, W. Giersch, Y. Niclass, G. Bernardinelli and G. Ohloff, Helv. Chim. Acta, 1990, 73, 1935 CrossRef CAS; (b) K. Mori, S. Aki and M. Kido, Liebigs Ann. Chem., 1994, 319 CrossRef CAS.
- A. Abad, C. Agullió, A. C. Cuñat, A. González-Coloma and A. Pardo, Eur. J. Org. Chem., 2010, 2182 CrossRef CAS.
- The treatment of ketoester 10 with 98% aq. H2SO4 afforded a complex mixture of compounds, including a C-alkylation hydroxy ester and a mixture of spiro enol ether isomers in low proportion. See: S. M. Linder, D. Reichlin, D. P. Simmons and R. L. Snowden, Tetrahedron Lett., 1993, 34, 4789 CrossRef CAS.
- (a) M. T. Reetz, I. Chatziosifidis and C. K. Schwellnus, Angew. Chem., Int. Ed. Engl., 1981, 20, 687 CrossRef; (b) E. E. Van Tamelen, J. R. Hwu and T. M. Leiden, J. Chem. Soc., Chem. Commun., 1983, 62 RSC.
- (a) D. Yang, J.-H. Li, Q. Gao and Y.-L. Yan, Org. Lett., 2003, 5, 2869 CrossRef CAS PubMed; (b) X. Han, X. Wang, T. Pei and R. A. Widenhoefer, Chem.–Eur. J., 2004, 10, 6333 CrossRef CAS PubMed.
- The results obtained by treating β-ketoesters 9, 10, 12 and 13 with different cyclizing reagents are included in the ESI†.
- E. Alvarez-Manzaneda, R. Chahboun, E. Cabrera Torres, E. Alvarez, R. Alvarez-Manzaneda, A. Haidour and J. Ramos, Tetrahedron Lett., 2004, 45, 4453 CrossRef CAS; E. Alvarez-Manzaneda, R. Chahboun, E. Cabrera Torres, E. Alvarez, R. Alvarez-Manzaneda, A. Haidour and J. Ramos, Tetrahedron Lett., 2005, 46, 1075 CrossRef; E. Alvarez-Manzaneda, R. Chahboun, E. Cabrera Torres, E. Alvarez, R. Alvarez-Manzaneda, A. Haidour and J. Ramos, Tetrahedron Lett., 2005, 46, 3755 CrossRef.
- Compounds 12, 13 and 16 were obtained after methoxycarbonylation or formylation of the corresponding methylketones. See: (a) K. Mori and Y. Koga, Liebigs Ann. Chem., 1991, 769 CrossRef CAS; (b) E. Alvarez-Manzaneda, R. Chahboun, E. Alvarez, A. Fernández, R. Alvarez-Manzaneda, A. Haidour, J. M. Ramos and A. Akhaouzan, Chem. Commun., 2012, 48, 606 RSC.
- Compound 14 was synthesized from abietic acid. R. Tapia, PhD thesis, University of Granada, 2012.
- T. Narender, S. Sarkar, K. Venkateswarlu and J. K. Kumar, Tetrahedron Lett., 2010, 51, 6576 CrossRef CAS.
- H. Monti, N. Tiliacos and R. Faure, Phytochemistry, 1996, 42, 1653 CrossRef CAS.
- J. Justicia, A. Rosales, E. Buñuel, J. L. Oller-López, M. Valdivia, A. Haidour, J. E. Oltra, A. F. Barrero, D. Cárdenas and J. M. Cuerva, Chem.–Eur. J., 2004, 10, 1778 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures, spectroscopic data and copies of 1H and 13C NMR spectra. See DOI: 10.1039/c3cc45921g |
| This journal is © The Royal Society of Chemistry 2013 |