Open Access Article
Open Access ArticleWater-soluble C60– and C70–PVP polymers for biomaterials with efficient 1O2 generation†
Sean Orianaa,
Safwan Arouaa,
Justus Oliver Butz Söllnera,
Xiao-Jing Ma‡a,
Yuko Iwamotoa and
Yoko Yamakoshi*ab
aLaboratorium für Organische Chemie, ETH-Zürich, Wolfgang-Pauli-Strasse 10, Ch-8093 Zürich, Switzerland. E-mail: yamakoshi@org.chem.ethz.ch; Fax: +41 44 633 1235; Tel: +41 44 633 6420
bDepartment of Radiology, University of Pennsylvania, Philadelphia PA 19104, USA
First published on 14th August 2013
Abstract
Highly water-soluble fullerene polymers were successfully prepared by a simple direct free-radical copolymerization of N-vinylpyrrolidone and intact C60 or C70 as a radical-capping agent. Using AIBN as a radical initiator, the polymers (C60– or C70–PVP) with significantly high molecular weight (∼30 kDa) and with efficient 1O2 generation were obtained.
The bioapplications of fullerenes have been discussed for more than a decade due to their high photosensitivity and metal encapsulation ability. The main obstacle to developing fullerene biomaterials is their extremely low solubility in water or water-miscible solvents. Following the initial studies on water-soluble C60 derivatives,1–5 various water-soluble materials were reported as biocompatible fullerenes6–9 addressing photodynamic therapy (PDT) and MRI contrast enhancement agents. Recent attention in this field has been directed towards polymeric fullerene materials.10 For targeting inflammatory diseases such as cancers, where vascular leakage is often limited, polymeric materials (Mw > 20 kDa) are selectively accumulated due to the enhanced permeation and retention (EPR) effect. Previously, we have reported that water-soluble fullerene biomaterials in combination with biocompatible poly(vinylpyrrolidone) (PVP) can be used for preparing complexes11 or copolymers.12 In this study, we aimed to develop a versatile procedure for preparation of fullerene (C2n)–PVP copolymers using radical polymerization of N-vinylpyrrolidone (NVP) in the presence of intact fullerenes that react with ˙PVP on their double bonds to form a covalent bond (Fig. 1). This procedure does not require the preparation of fullerene monomer derivatives (e.g. C2n-vinyl derivatives) and will allow easy access to the preparation of water-soluble higher fullerenes such as C70 and Gd3N@C80 with biologically attractive properties including a longer lifetime of the triplet excited state and paramagneticity, respectively. There are only few studies on water-soluble materials of such higher fullerenes. And particularly with C70, similar reactions are expected to occur as radical addition reactions are known.13
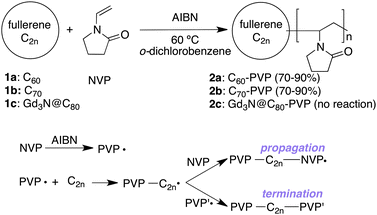 | ||
Fig. 1 Preparation of fullerene–PVP copolymers by the direct free-radical copolymerization of NVP and C60, C70, or Gd3N@C80. AIBN (0.075–0.25 equiv.) was used as a radical initiator. The initial ratio of C2n and NVP considered in the reaction was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 200, 300, 400, or 500. 100, 200, 300, 400, or 500. | ||
Studies on such direct free-radical copolymerization reactions of fullerenes involving intact C60 were initially reported as C60–poly(styrene) copolymers.14–18 In those studies, AIBN was used as a radical initiator and the poly(styrene) radical generated in situ was speculated to attack the double bonds of C60 forming covalent bonds. A similar procedure was used to prepare other polymers such as C60–poly(carbonate)19 and C60–poly(methylmethacrylate).20,21 Conjugation with controlled polymers generated by nitroxide-mediated radical polymerization was also reported.22–24 However, direct radical copolymerization of intact C60 and NVP was not investigated except in a few studies using lauroyl peroxide or benzoyl peroxide as the radical initiators. However, these results provided low yields and insufficient water-solubility.25,26
In the present study, we tested several different ratios of NVP (100–500 equiv.) to C2n to optimize the conditions for the preparation of C2n–PVP polymers with a sufficiently large molecular weight and high water-solubility. C60, C70, and Gd3N@C80 were used as fullerene cores for polymerization with AIBN as a radical initiator (Fig. 1). Considering the radical quenching activities of fullerenes, which often disturb radical polymerization,27,28 a relatively large amount of AIBN (0.075–0.25 equiv. of NVP, 40 equiv. of C2n) was added to each reaction.29 The reactions were carried out in o-dichlorobenzene, a good solvent for fullerenes.
All co-polymerization reactions of C60 or C70 with NVP provided fullerene–PVP materials in sufficient yields (>70%, Table 1, column 3). However, the reaction of Gd3N@C80 and NVP did not provide any polymeric product. We speculated that the radical quenching activity, which is common to many fullerenes, was too high in Gd3N@C80 to provide any polymers (further details need to be clarified). C60–PVP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–500) and C70–PVP (1
100–500) and C70–PVP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200–500) were thoroughly soluble in water. Only the C70–PVP (1
200–500) were thoroughly soluble in water. Only the C70–PVP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) polymer was not soluble in water presumably due to the larger hydrophobic surface area of C70. The molecular weight of each copolymer (C60– or C70–PVP) was analyzed by GPC (solvent: DMF, calibration standard: poly(methylmethacrylate) (PMMA)). Interestingly, the Mw of C2n–PVP polymers were highly correlated with the ratios of C2n and NVP. While the reaction with a higher ratio of NVP (lower ratio of C2n) provided polymers with higher Mw, the reaction with the lower ratio of NVP provided polymers with lower Mw (Table 1, columns 2 and 4). This result suggests that C60 or C70 are acting as an end-capping reagent of ˙PVP (Fig. 1) and upon increasing the ratio of NVP, the PVP–C2n radical would have a higher possibility of reacting with NVP, leading to the higher Mw value. To ensure that the fullerene cores were covalently bound to the polymer (not by complexation), aqueous polymer solutions were extracted with toluene in the presence of oversaturated NaCl. No fullerene was detected in the organic layer indicating that C2n materials were water-soluble due to covalent functionalization.
100) polymer was not soluble in water presumably due to the larger hydrophobic surface area of C70. The molecular weight of each copolymer (C60– or C70–PVP) was analyzed by GPC (solvent: DMF, calibration standard: poly(methylmethacrylate) (PMMA)). Interestingly, the Mw of C2n–PVP polymers were highly correlated with the ratios of C2n and NVP. While the reaction with a higher ratio of NVP (lower ratio of C2n) provided polymers with higher Mw, the reaction with the lower ratio of NVP provided polymers with lower Mw (Table 1, columns 2 and 4). This result suggests that C60 or C70 are acting as an end-capping reagent of ˙PVP (Fig. 1) and upon increasing the ratio of NVP, the PVP–C2n radical would have a higher possibility of reacting with NVP, leading to the higher Mw value. To ensure that the fullerene cores were covalently bound to the polymer (not by complexation), aqueous polymer solutions were extracted with toluene in the presence of oversaturated NaCl. No fullerene was detected in the organic layer indicating that C2n materials were water-soluble due to covalent functionalization.
| Reagents considereda | Properties of the obtained polymer | |||||
|---|---|---|---|---|---|---|
| C2n | Ratio of NVP to C2n | Yield [%] | Mwb [kDa] | PDI | Particle sizec [nm] | |
| Fresh | 1-day old | |||||
| a AIBN with 0.25–0.075 equiv. to NVP was used.b Analyzed by GPC. The chromatogram is shown in Fig. S24 and S25 (ESI).c Analyzed by DLS (mean). Shown in detail in Fig. S26 (ESI).d Not soluble in water. | ||||||
| C60 | 100 | 71 | 10.9 | 1.75 | 6.3/27.6 | 6.2/23.2 |
| 200 | 93 | 13.3 | 1.75 | 8.02 | 4.94 | |
| 300 | 74 | 13.3 | 1.76 | 5.9/15.8 | 5.1 | |
| 400 | 72 | 18.6 | 1.86 | 5.3 | 5.7 | |
| 500 | 73 | 32.5 | 1.96 | 6.6 | 6.5 | |
| C70 | 100d | 85 | — | — | — | — |
| 200 | 73 | 7.9 | 1.76 | 5.3/24.9 | 6.6/21.7 | |
| 300 | 87 | 15.3 | 1.86 | 5.0 | 4.8 | |
| 400 | 71 | 19.4 | 2.38 | 5.5 | 5.8 | |
| 500 | 70 | 29.7 | 2.47 | 6.9 | 7.0 | |
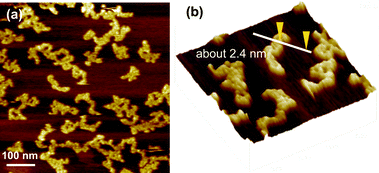
The particle size was analyzed by dynamic laser scattering (DLS) using both freshly prepared and 1-day-old solutions. The distribution of particle size of polymers generally became uniform after keeping the solutions in water at room temperature for 1-day with the shift to the smaller particle size ranges (Fig. S26, ESI†). Despite the difference in Mw values, the sizes of the polymer particle measured by DLS were not significantly different, presumably because the polymers are not globular in water. The AFM image of the C60–PVP polymer supports this speculation and showed that the polymers formed a linear bundled shape rather than the micellar form (Fig. 2). The distribution of particle size was narrower in the polymers with higher ratios of NVP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400–500) presumably due to the longer hydrophilic PVP part that helps in the formation of uniform C2n–PVP particles in the aqueous solution.
400–500) presumably due to the longer hydrophilic PVP part that helps in the formation of uniform C2n–PVP particles in the aqueous solution.
Solubility curves of the C60– and C70–PVP polymers are shown in Fig. 3a and b. As calculated based on the molar concentration of C2n, the solubility of all of the polymers were in the same range (∼10 to 15 mM for both C60– and C70–PVP, Fig. S22 and S23, ESI†) and were sufficient for many bio-applications.
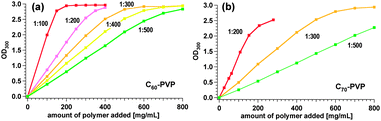 | ||
| Fig. 3 Solubility curves of C60– (a) and C70–PVP (b) in water. To an aliquot of each polymer, Milli-Q water was added and subsequently filtered. The filtrates were diluted with Milli-Q water before the absorption measurements. | ||
As one of the evaluations of C2n–PVP polymers as PDT agents, reactive oxygen species (ROS) generated under visible light irradiation were estimated using ESR spin-trapping methods (Fig. 4). In general, C60 and C70 can be excited by visible light irradiation to generate either singlet oxygen (1O2) or superoxide radical anions (O2˙−) via an energy transfer type II pathway or via an electron transfer type I pathway, respectively (Scheme 1).
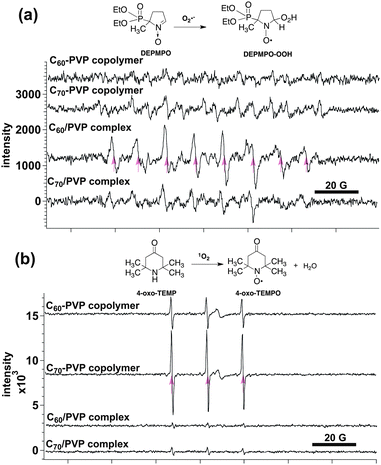 | ||
| Fig. 4 ROS generation of C60– and C70–PVP copolymers under visible light irradiation and in the aqueous solution. (a) O2˙− generation trapped with DEPMPO (200 W photoreflector lamp, 3 min, in the presence of NADH as an electron donor). (b) 1O2 generation trapped with 4-oxo-TEMP (200 W photoreflector lamp, 5 min). Arrows correspond to the signals of the adducts of ROS and spin-trapping agents. C60– and C70–PVP complexes were used in the control experiment. | ||
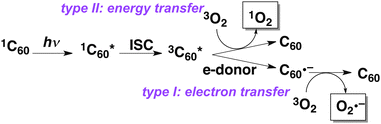 | ||
| Scheme 1 Types I and II pathways of ROS generation from photoexcited C60. | ||
According to the previous studies,30,31 including ours,32 1O2 generation from photoexcited C60 or C70 was generally observed in less polar solvents (e.g. benzene) and not in the aqueous solution. Instead, O2˙− is often detected in aqueous solution in the presence of electron donors with a physiological concentration. This is shown in the control experiments using C60– or C70–PVP complexes as a DEPMPO–OOH signal in Fig. 4a (3rd and 4th lines). However in our present study, no O2˙− generation from aqueous solutions of C60– and C70–PVP copolymers was detected in the presence of an electron donor. Alternatively, 1O2 generation was clearly observed as a signal of 4-oxo-TEMPO, a 1O2 adduct of 4-oxo-TEMP (Fig. 4b, the C60– or C70–PVP copolymer). This result clearly shows that type II energy transfer reaction of 3C60* and 3C70* is favoured in C2n–PVP copolymer solution even in polar media (Scheme 1). Interestingly, the 1O2 generation from C70–PVP was higher than that from C60–PVP, which may be related to the longer lifetime of the triplet excited state of C70 (130 μs)31 compared to C60 (40 μs).30 Although the detailed mechanism of ROS generation should be investigated further, such efficient generation of ROS from C60– and C70–PVP shows their high potential as biocompatible polymer PDT agents.
In summary, the C60– and C70–PVP copolymers were successfully prepared via an easy-to-handle polymerization method of NVP using C60 or C70 as a terminal group. The ratio of C2n and NVP can efficiently affect the size of the polymers. The obtained polymers showed high water-solubility and efficient 1O2 generation showing high potential as a PDT agent. A detailed mechanistic study of 1O2 generation is in progress.
The authors thank Prof. Dr M. Morbidelli and Prof. Dr N. Spencer (ETH-Zürich) for their help in DLS and AFM measurements. The authors thank Profs Drs E. Ogiso-Nakamaru and T. Ohnishi (University of Pennsylvania) for their help in ESR measurements. This research was supported in part by the ETH Research Grant, Swiss National Foundation, PRESTO program from JST, the Sino Swiss Science and Technology Cooperation (SSSTC) Program, CEET pilot grant from the University of Pennsylvania and American Heart Association Researcher's Developing Grant (YY).
Notes and references
- H. Tokuyama, S. Yamago, E. Nakamura, T. Shiraki and Y. Sugiura, J. Am. Chem. Soc., 1993, 115, 7918–7919 CrossRef CAS.
- M. Prato, A. Bianco, M. Maggini, G. Scorrano, C. Toniolo and F. Wudl, J. Org. Chem., 1993, 58, 5578–5580 CrossRef CAS.
- R. Sijbesma, G. Srdanov, F. Wudl, J. A. Castoro, C. Wilkins, S. H. Friedman, D. L. Decamp and G. L. Kenyon, J. Am. Chem. Soc., 1993, 115, 6510–6512 CrossRef CAS.
- K. L. Wooley, C. J. Hawker, J. M. J. Frechet, F. Wudl, G. Srdanov, S. Shi, C. Li and M. Kao, J. Am. Chem. Soc., 1993, 115, 9836–9837 CrossRef CAS.
- I. Lamparth and A. Hirsch, J. Chem. Soc., Chem. Commun., 1994, 1727–1728 RSC.
- M. Prato, J. Mater. Chem., 1997, 7, 1097–1109 RSC.
- E. Nakamura and H. Isobe, Acc. Chem. Res., 2003, 36, 807–815 CrossRef CAS PubMed.
- T. Da Ros and M. Prato, Chem. Commun., 1999, 663–669 RSC.
- S. Bosi, T. Da Ros, G. Spalluto and M. Prato, Eur. J. Med. Chem., 2003, 38, 913–923 CrossRef CAS PubMed.
- F. Giacalone and N. Martin, Adv. Mater., 2010, 22, 4220–4248 CrossRef CAS PubMed.
- Y. Yamakoshi, T. Yagami, K. Fukuhara, S. Sueyoshi and N. Miyata, J. Chem. Soc., Chem. Commun., 1994, 517–518 RSC.
- Y. Iwamoto and Y. Yamakoshi, Chem. Commun., 2006, 4805–4807 RSC.
- R. Borghi, L. Lunazzi, G. Placucci, P. J. Krusic, D. A. Dixon and L. B. Knight, J. Phys. Chem., 1994, 98, 5395–5398 CrossRef CAS.
- T. Cao and S. E. Webber, Macromolecules, 1995, 28, 3741–3743 CrossRef CAS.
- C. E. Bunker, G. E. Lawson and Y. P. Sun, Macromolecules, 1995, 28, 3744–3746 CrossRef CAS.
- A. G. Camp, A. Lary and W. T. Ford, Macromolecules, 1995, 28, 7959–7961 CrossRef CAS.
- T. Cao and S. E. Webber, Macromolecules, 1996, 29, 3826–3830 CrossRef CAS.
- Y. P. Sun, G. E. Lawson, C. E. Bunker, R. A. Johnson, B. Ma, C. Farmer, J. E. Riggs and A. Kitaygorodskiy, Macromolecules, 1996, 29, 8441–8448 CrossRef CAS.
- B. Z. Tang, S. M. Leung, H. Peng, N. T. Yu and K. C. Su, Macromolecules, 1997, 30, 2848–2852 CrossRef CAS.
- W. T. Ford, T. D. Graham and T. H. Mourey, Macromolecules, 1997, 30, 6422–6429 CrossRef CAS.
- W. T. Ford, T. Nishioka, S. C. McCleskey, T. H. Mourey and P. Kahol, Macromolecules, 2000, 33, 2413–2423 CrossRef CAS.
- H. Okamura, T. Terauchi, M. Minoda, T. Fukuda and K. Komatsu, Macromolecules, 1997, 30, 5279–5284 CrossRef CAS.
- H. Okamura, N. Ide, M. Minoda, K. Komatsu and T. Fukuda, Macromolecules, 1998, 31, 1859–1865 CrossRef CAS.
- W. T. Ford, A. L. Lary and T. H. Mourey, Macromolecules, 2001, 34, 5819–5826 CrossRef CAS.
- E. Rusen, B. Marculescu, N. Preda, C. Bucur and L. Mihut, Polym. Bull., 2008, 61, 581–592 CrossRef CAS.
- G. C. Jiang and G. T. Li, Biotechnol. Prog., 2012, 28, 215–222 CrossRef CAS PubMed.
- D. Stewart and C. T. Imrie, Chem. Commun., 1996, 1383–1384 RSC.
- M. Seno, H. Fukunaga and T. Sato, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 2905–2912 CrossRef CAS.
- Preliminary condition with smaller amount of AIBN (4 equiv. to fullerenes) did not provide any polymer product.
- J. W. Arbogast, A. P. Darmanyan, C. S. Foote, Y. Rubin, F. N. Diederich, M. M. Alvarez, S. J. Anz and R. L. Whetten, J. Phys. Chem., 1991, 95, 11–12 CrossRef CAS.
- J. W. Arbogast and C. S. Foote, J. Am. Chem. Soc., 1991, 113, 8886–8889 CrossRef CAS.
- Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, T. Masumizu and T. Nagano, J. Am. Chem. Soc., 2003, 125, 12803–12809 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details of polymerization characterization data of polymers (1H-NMR, UV-Vis, FT-IR, DLS, ESR). See DOI: 10.1039/c3cc45501g |
| ‡ Present address: State Key Lab of Polymer Physics and Chemistry, Changchun Inst. Appl. Chem., Chinese Academy of Sciences, China. |
| This journal is © The Royal Society of Chemistry 2013 |