Open Access Article
Open Access ArticleHypervalent iodine(III)-induced oxidative [4+2] annulation of o-phenylenediamines and electron-deficient alkynes: direct synthesis of quinoxalines from alkyne substrates under metal-free conditions†
Sota Okumuraa,
Youhei Takeda*ab,
Kensuke Kiyokawaa and
Satoshi Minakata*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan. E-mail: minakata@chem.eng.osaka-u.ac.jp; Fax: +81-6-6879-7402; Tel: +81-6-6879-7400
bFrontier Research Base for Global Young Researchers, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan. E-mail: takeda@chem.eng.osaka-u.ac.jp
First published on 14th August 2013
Abstract
Hypervalent iodine(III)-induced oxidative [4+2] annulation of o-phenylenediamines and electron-deficient alkynes under metal-free conditions has been developed. The reaction allows for direct access to quinoxalines bearing two electron-withdrawing groups in an efficient manner.
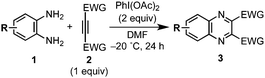
Quinoxaline derivatives not only constitute an important class of biologically active agents,1 but also find tremendous applications in materials science such as luminescent materials2 and low-band-gap polymers.3 Of the reported methods,4 the most widely used approach involves condensation of o-phenylenediamines with 1,2-dicarbonyl compounds bearing electron-rich or neutral substituents, which are generally prepared by oxidation of upstream alkynes (Scheme 1a). On the other hand, the synthetic methods of the quinoxalines bearing electron-withdrawing groups (EWGs, e.g., –COR, –CO2R, –SO2R) have been poorly explored,5 although such compounds can serve as promising candidates for (opto)electronic materials5a,6 and as versatile synthetic intermediates. Herein we present a hypervalent iodine(III) reagent-induced oxidative [4+2] annulation of o-phenylenediamines and electron-deficient alkynes (Scheme 1b), which allows for direct access to electron-deficient quinoxalines from alkynes instead of diketo substrates in an efficient manner.
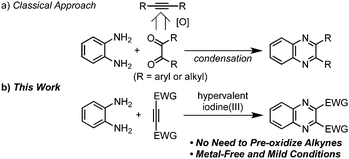 | ||
| Scheme 1 Synthetic approaches to quinoxalines. | ||
Recently, we have reported an oxidative dimerization of anilines through the agency of a unique and powerful iodinating reagent, tert-butyl hypoiodite (t-BuOI), leading to aromatic azo compounds in an efficient and selective manner.7 The key to the success is an efficient two-fold iodination of nitrogen-center, forming ArNI2 species which then serves as an electrophile to form N–N bonds. Based on the findings, we envisioned that a tandem process consisting of (i) the Michael-addition of o-phenylenediamine to an electron-deficient alkyne and (ii) a subsequent nucleophilic attack on the highly electrophilic N-center (NI2) by the resulting enamine could form a dihydro-quinoxaline skeleton. The subsequent elimination of HI would produce quinoxalines. At the outset, we attempted the oxidative annulation of o-phenylenediamine (1a) and DMAD (2a) as a model reaction (Table 1). However, contrary to our preliminary expectation, the results were disappointing: the treatment of an equimolar mixture of 1a and 2a with t-BuOI (4 equiv.) at −20 °C gave cis,cis-mucononitrile (5) as a major product, which should be formed through oxidative dearomatization and the following C–C bond cleavage of the benzene core (entries 1 and 2).8 These results clearly suggested that t-BuOI is not an appropriate oxidant for the aimed transformation, probably due to the rapid H–I exchange and dearomatization processes of 1a prior to Michael-addition. After extensive screening of iodine-containing reagents, we were delighted to find that the employment of phenyliodine diacetate (PIDA) was highly effective for the progression of the desired annulation (entries 3–6). It should be noted that protecting group-free phenylenediamines, which are usually labile to oxidation reactions, were applicable to the annulation. Hypervalent iodine(III) compounds have been emerging as powerful reagents in organic synthesis due to their diverse reactivity as well as to high-availability and environmentally-benign character.9 Specifically, PIDA and its derivatives have been utilized to develop privileged oxidative C–N bond forming reactions.10,11 Nonetheless, to the best of our knowledge, hypervalent iodine(III)-mediated oxidative annulation reaction that leads to quinoxaline, has not been reported to date.12 Intriguingly, a significant solvent effect was observed (entries 3–6): as the polarity of solvent increased, the yield of 3a was enhanced while that of the by-product 4a13 was decreased.14 Other representative hypervalent iodine(III) reagents were found ineffective for the annulation (entries 7–10), and PIDA was indispensable for the annulation reaction (entry 11).
|
||||||
|---|---|---|---|---|---|---|
| Entry | Oxidant (equiv.) | Solvent | T [°C] | Yieldb [%] | ||
| 3a | 4a | 5 | ||||
| a Reaction conditions: 1a (0.25 mmol), 2a (0.25 mmol), and iodine-containing oxidant (0.50–1.0 mmol) were mixed in a solvent (3 mL) at the temperature in the column and stirred for 24 h.b 1H NMR yields.c Isolated yield. | ||||||
| 1 | t-BuOI (4) | THF | −20 | 12 | 0 | 64 |
| 2 | t-BuOI (4) | DME | −20 | 2 | 0 | 33 |
| 3 | PhI(OAc)2 (2) | CH2Cl2 | −20 | 5 | 40 | 0 |
| 4 | PhI(OAc)2 (2) | THF | −20 | 63 | 18 | 0 |
| 5 | PhI(OAc)2 (2) | DME | −20 | 60c | 4c | 0 |
| 6 | PhI(OAc)2 (2) | DMF | −20 | 92c | 4c | 0 |
| 7 | PhI(OCOCF3)2 (2) | DMF | −20 | 3 | — | 0 |
| 8 | PhI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (2) O (2) |
DMF | −20 | 0 | — | 0 |
| 9 | 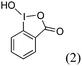 |
DMF | −20 | 0 | — | 0 |
| 10 | PhI(OH)OTs (2) | DMF | −20 | 0 | — | 0 |
| 11 | — | DMF | −20 | 0 | 21 | 0 |
Having optimized the reaction conditions, the scope of the annulation was investigated (Table 2). A wide variety of diamines bearing an electron-rich, -neutral, and -deficient substituent reacted with DMAD to give corresponding quinoxalines 3b–3j in high yields. Multiple-substituted diamines afforded 3k and 3l. Furthermore, sterically demanding diamines gave the corresponding product 3m in excellent yield. Although the reaction of naphthalene-2,3-diamine with DMAD required prolonged time, N-heteroacene 3n, which constitutes a family of electron-transporting materials,15 was obtained in 56% yield. Using the method, biquinoxaline 3o was prepared in good yield. In respect to alkyne substrates, dibutyl acetylenedicarboxylate was successfully applied to the reaction conditions to afford 3p and 3q in 77% and 44% yield, respectively. In addition, an unsymmetrical alkyne having an ester and a sulfonyl group also successfully underwent the annulation to give 3r in good yield.
|
|---|
| a Reaction conditions: 1 (0.25 mmol), 2 (0.25 mmol), and PhI(OAc)2 (0.50 mmol) were mixed in DMF (3 mL) at 20 °C and stirred for 24 h.b The values in parentheses indicate the yields of quinoxaline products.c Reaction time: 48 h.d [1,1′-biphenyl]-3,3′,4,4′-tetraamine (1o) (0.25 mmol), 2a (0.50 mmol), PhI(OAc)2 (1.0 mmol) were employed. |
 |
Taking advantage of the ester functionality, 3a was diversely derivatized into functionalized quinoxalines (Scheme 2). For example, diester of 3a easily underwent hydrolysis to give dicarboxylic acid 6 in high yield, which was further efficiently converted to 7 by dehydration. Moreover, anhydride 7 was successfully transformed into imide-fused quinoxaline 8 by condensation with p-toluidine, which is an N-analogue of triboluminescent material.16 It is noted that such compounds are quite difficult to prepare by traditional condensation methods.17
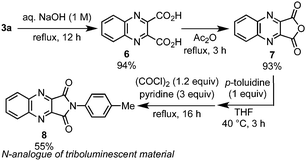 | ||
| Scheme 2 Derivatization of 3a. | ||
To investigate the reaction pathways, several experiments were conducted as follows: enamine 9, which was readily prepared by the Michael-addition of N-Boc-protected o-phenylenediamine to DMAD,13 was treated with PIDA in the presence of trifluoroacetic acid (Scheme 3).18 At −20 °C, 9 underwent oxidative cyclization to give N-Boc dihydroquinoxaline 10 in 45% yield,19 while 9 was quantitatively recovered in the absence of PIDA. In contrast, at room temperature, quinoxaline 3a was obtained in 54% yield. In conjunction with the fact that DMAD does not react with PIDA in the absence of o-phenylenediamine, the most likely intermediate of the annulation would be the deprotected counterpart of the Michael-adduct 9 as preliminary assumed.
 | ||
| Scheme 3 Oxidative cyclization of enamine 9. | ||
On the basis of the experimental results and knowledge accumulated from the literature about hypervalent iodine(III)-mediated oxidative C–N bond forming reactions using enamine substrates,20–22 conceivable reaction pathways are illustrated in Scheme 4. The reaction would start with Michael addition of o-phenylenediamine to DMAD, forming the enamine intermediate A, which has three possible reactive points when reacting with PIDA, namely, β-carbon of enamine (a),20 enamine nitrogen (b),21 and nitrogen on the benzene moiety (c).22 Accordingly, three species should be extrapolated as intermediates prior to cyclization: (i) α-iodo(III) imine B (route a); (ii), (iii) enamines C and D (routes b and c, respectively). Successive cyclizative nucleophilic substitution on the iodine-attached sp3-carbon (from B), on the enamine carbon in a pseudo-SN2′ manner (from C), or on the electrophilic N-center (from D) would provide a common intermediate E. Oxidative aromatization of E with another equivalent of PIDA should lead to quinoxaline F. Ma and Lei reported an oxidative dimerization of aromatic amines using PIDA to give azobenzenes.22 No azo compounds were detected in our system, suggesting that the pathway via intermediacy of D (route c) might be excluded. On the one hand, according to the reactivity of carbonyl-conjugated enamines (i.e., enaminones),23 electrophilic reagents, including iodine electrophiles such as BTMA·ICl2,24a I(Py)2BF4,24b and CF3CH2I(OH)(OTs),24c react exclusively at the enamine β-carbon. Taken together, we believe that the most likely reaction pathway is route a, although routes b, c, and other possible pathways cannot be excluded completely.25
 | ||
| Scheme 4 Conceivable reaction pathways. | ||
In summary, a simple, efficient and metal-free synthesis of electron-deficient quinoxalines through oxidative annulation of o-phenylenediamines and alkynes has been developed. Further investigations into the mechanism and application to the construction of functional materials are currently underway in our laboratory.
This research was partly supported by a research Grant from the Ogasawara Foundation for the Promotion of Science & Engineering (to Y.T.), by a Grant-in-Aid for Scientific Research (B) from the JSPS, Japan (No. 25288047, to S.M.), and by a research grant from the Nagase Science and Technology Foundation (to S.M.). Also, Y.T. acknowledges all support from the Frontier Research Base for Global Young Researchers, Osaka University, from the Program of MEXT.
Notes and references
- (a) L. Yan, F.-W. Liu, G.-F. Dai and H.-M. Liu, Bioorg. Med. Chem. Lett., 2007, 17, 609 CrossRef CAS PubMed; (b) L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2002, 45, 5604 CrossRef CAS PubMed.
- S. Achelle, C. Baudequin and N. Plé, Dyes Pigm., 2013, 98, 575 CrossRef CAS PubMed.
- Y. Zhang, J. Zou, H.-L. Yip, K.-S. Chen, D. F. Zeigler, Y. Sun and A. K.-Y. Jen, Chem. Mater., 2011, 23, 2289 CrossRef CAS and references therein.
- For reviews of synthetic methods of quinoxalines, see: (a) D. F. Saifina and V. A. Mamedov, Russ. Chem. Rev., 2010, 79, 351 CrossRef CAS PubMed; (b) G. Sakata, K. Makino and Y. Kurasawa, Heterocycles, 1988, 27, 2481 CrossRef CAS PubMed.
- (a) P. Gawrys, T. Marszalek, E. Bartnik, M. Kucinska, J. Ulanski and M. Zagorska, Org. Lett., 2011, 13, 6090 CrossRef CAS PubMed; (b) T. M. V. D. Pinho e Melo, C. S. J. Lopes, A. M. d'A. Rocha Gonsalves, A. M. Beja, J. A. Paixão, M. R. Silva and L. A. da Veiga, J. Org. Chem., 2001, 67, 66 CrossRef PubMed; (c) H. W. Rothkopf, D. Wöhrle, R. Müller and G. Koßmehl, Chem. Ber., 1975, 108, 875 CrossRef CAS.
- Q. Tang, Z. Liang, J. Liu, J. Xu and Q. Miao, Chem. Commun., 2010, 46, 2977 RSC.
- (a) Y. Takeda, S. Okumura and S. Minakata, Synthesis, 2013, 1029 CAS; (b) Y. Takeda, S. Okumura and S. Minakata, Angew. Chem., Int. Ed., 2012, 51, 7804 CrossRef CAS PubMed.
- (a) V. N. Telvelar and H. M. Bachhav, Synlett, 2009, 2059 Search PubMed; (b) V. N. Telvekar and B. S. Takale, Tetrahedron Lett., 2010, 51, 3940 CrossRef CAS PubMed.
- (a) T. Dohi and Y. Kita, Chem. Commun., 2009, 2073 RSC; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (c) R. M. Moriarty, J. Org. Chem., 2005, 70, 2893 CrossRef CAS PubMed; (d) T. Wirth, Angew. Chem., Int. Ed., 2005, 44, 3656 CrossRef CAS PubMed; (e) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS PubMed.
- For a review, see: M. A. Ciufolini, N. A. Braun, S. Canesi, M. Ousmer, J. Chang and D. Chai, Synthesis, 2007, 3759 CrossRef CAS PubMed.
- For recent examples, see: (a) J. A. Souto, D. Zian and K. Muñiz, J. Am. Chem. Soc., 2012, 134, 7242 CrossRef CAS PubMed; (b) C. Röben, J. A. Souto, Y. González, A. Lishchynskyi and K. Muñiz, Angew. Chem., Int. Ed., 2011, 50, 9478 CrossRef PubMed; (c) A. A. Kantak, S. Potavathri, R. A. Barham, K. M. Romano and B. DeBoef, J. Am. Chem. Soc., 2011, 133, 19960 CrossRef CAS PubMed; (d) S. Hwan, J. Yoon and S. Chang, J. Am. Chem. Soc., 2011, 133, 5996 CrossRef PubMed; (e) A. P. Antonchick, R. Samanta, K. Kulikov and J. Lategahn, Angew. Chem., Int. Ed., 2011, 50, 8605 CrossRef CAS PubMed; (f) Y. Du, R. Liu, G. Linn and K. Zhao, Org. Lett., 2006, 8, 5919 CrossRef CAS PubMed; (g) M. Ousmer, N. A. Braun, C. Bavoux, M. Perrin and M. A. Ciufolini, J. Am. Chem. Soc., 2001, 123, 7534 CrossRef CAS PubMed.
- Two-step synthesis of quinoxalines through hypervalent iodine(III)-mediated oxidation of alkynes and condensation of the resultant diketones with diamines has been reported: M. Tingoli, M. Mazzella, B. Panunzi and A. Tuzi, Eur. J. Org. Chem., 2011, 399 CrossRef CAS.
- G. Choudhary and R. K. Peddinti, Green Chem., 2011, 13, 3290 RSC.
- For the detailed explanation, see the ESI†.
- U. H. F. Bunz, J. U. Engelhart, B. D. Lindner and M. Schaffroth, Angew. Chem., Int. Ed., 2013, 52, 3810 CrossRef CAS PubMed.
- H. Nakayama, J. Nishida, N. Takada, H. Sato and Y. Yamashita, Chem. Mater., 2012, 24, 671 CrossRef CAS.
- L. Hanaineh-Abdelnour, S. Bayyuk and R. Theodorie, Tetrahedron, 1999, 55, 11859 CrossRef CAS.
- Although we have tried preparation of the Michael-adduct starting from 1a (deprotection form of 9) by a similar method reported in ref. 13, it failed only to produce 4a alone. Trifluoroacetic acid was added for the purpose of detaching the Boc group of resulting intermediates.
- The deprotected counterpart of 9 was not formed at all.
- P. Gao, J. Liu and Y. Wei, Org. Lett., 2013, 15, 2872 CrossRef CAS PubMed.
- (a) W. Liu, C. Chen and Q. Zhang, Org. Biomol. Chem., 2011, 9, 6484 RSC; (b) W. Liu, H. Jiang and L. Huang, Org. Lett., 2010, 12, 312 CrossRef CAS PubMed; (c) J.-Y. Wang, S.-P. Liu and W. Yu, Synlett, 2009, 2529 Search PubMed; (d) X. Li, Y. Du, Z. Liang, X. Li, Y. Pan and K. Zhao, Org. Lett., 2009, 11, 2643 CrossRef CAS PubMed; (e) W. Yu, Y. Du and K. Zhao, Org. Lett., 2009, 11, 2417 CrossRef CAS PubMed.
- H. Ma, W. Li, J. Wang, G. Xiao, Y. Gong, C. Qi, Y. Feng, X. Li, Z. Bao, Q. Cao, Q. Sun, C. Veaceslav, F. Wang and Z. Lei, Tetrahedron, 2012, 68, 8358 CrossRef CAS PubMed.
- (a) G. Negri, C. Kascheres and A. J. Kascheres, J. Heterocycl. Chem., 2004, 41, 461 CrossRef CAS; (b) A.-Z. A. Elassar and A. A. El-Khair, Tetrahedron, 2003, 59, 8463 CrossRef CAS; (c) J. V. Greenhill, Chem. Soc. Rev., 1977, 6, 277 RSC.
- (a) C. P. Kordik and A. B. Reitz, J. Org. Chem., 1996, 61, 5644 CrossRef CAS; (b) P. J. Campos, J. Arranz and M. A. Rodriguez, Tetrahedron Lett., 1997, 38, 8397 CrossRef CAS; (c) I. Papoutsis, S. Spyroudis, A. Varvoglis, J. A. Callies and V. V. Zhdankin, Tetrahedron Lett., 1997, 38, 8401 CrossRef CAS.
- Diels–Alder reaction of oxidatively generated 1,2-diimines and DMAD might be possible, although the matching of the frontier orbitals of these substrates are less likely to be favorable.
Footnote |
| † Electronic supplementary information (ESI) available: Procedure for the synthesis and experimental data for quinoxalines and NMR spectra. See DOI: 10.1039/c3cc45469j |
| This journal is © The Royal Society of Chemistry 2013 |