Synthesis of biaryl imino/keto carboxylic acids via aryl amide directed C–H activation reaction†
Abstract
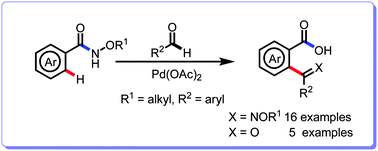
A novel Pd-catalysed C–H activation reaction for the synthesis of biaryl imino/keto carboxylic acids is developed. This reaction underwent aryl amide directed C–H activation ortho-acylation followed by ring closing and ring opening processes to give a range of biaryl imino/keto carboxylic acids. Our methodology features the utilization of a cheap and green oxidant (TBHP) as well as readily available aldehydes.

 Please wait while we load your content...
Please wait while we load your content...