 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lanthanide cation-templated synthesis of rotaxanes†
Fabiola
Zapata
,
Octavia A.
Blackburn
,
Matthew J.
Langton
,
Stephen
Faulkner
and
Paul D.
Beer
*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, Mansfield Road, Oxford, OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk; Fax: +44 (0)1865 272 690
First published on 1st August 2013
Abstract
The first lanthanide cation-templated synthesis of an interlocked structure is demonstrated through an interpenetrated assembly between a pyridine N-oxide threading component coordinating to a lanthanide cation complexed within a macrocycle. Stoppering of the pseudo-rotaxane assembly allows for preparation of the [2]rotaxane.
The development of new innovative strategic templating routes for the construction of mechanically interlocked molecules with potential molecular switching and machine-like nanotechnological applications is of intense current research interest.1 In addition to their dynamic behaviour, rotaxanes and catenanes can also be designed to contain topologically unique three-dimensional cavities for molecular recognition and sensing applications.2 Although transition metal cation, and to a lesser extent alkali and alkaline earth metal cation, templates have been imaginatively exploited in the synthesis of rotaxanes and catenanes,3 to the best of our knowledge, the utilization of lanthanides as potential templating reagents for interlocked molecular framework assembly has not been demonstrated.4 This is somewhat surprising given the usefulness of luminescent lanthanide complexes in imaging and assay.5 We describe herein the first lanthanide-cation templated synthesis of a rotaxane.
Adapting our recently reported sodium and barium cation templated synthesis of a rotaxane using the pyridine N-oxide motif,6 the synthetic strategy undertaken for the preparation of the target lanthanide-[2]rotaxane is shown in Scheme 1. A kinetically stable lanthanide complexed DOTA cyclen derivative integrated into a macrocyclic structural framework, forms initially a pseudo-rotaxane assembly with an appropriately functionalised pyridine N-oxide threading component, where the pyridine N-oxide ligand serves to satisfy the lanthanide cation's coordination sphere. Subsequent stoppering of the interpenetrated assembly produces the novel lanthanide containing interlocked structure.
![Schematic representation for the preparation of the target lanthanide-[2]rotaxane.](/image/article/2013/CC/c3cc45404e/c3cc45404e-s1.gif) | ||
| Scheme 1 Schematic representation for the preparation of the target lanthanide-[2]rotaxane. | ||
The synthetic procedure used for preparation of the lanthanide macrocyclic component 4a and 4b of the target [2]rotaxane system and the bis-azide functionalised pyridine N-oxide thread component is outlined in Scheme 2.
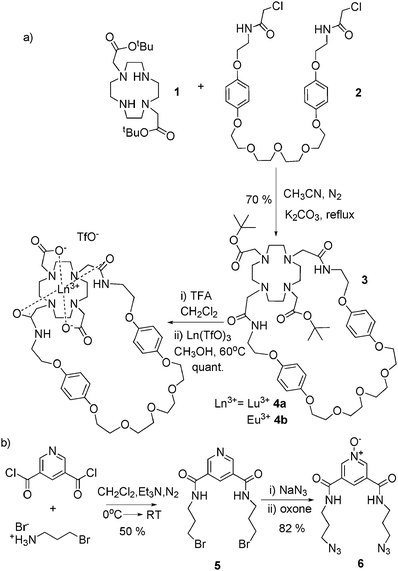 | ||
| Scheme 2 Synthesis of (a) lanthanide macrocyclic complexes and (b) bis-azide pyridine N-oxide thread component. | ||
Two of the nitrogen atoms of cyclen were selectively alkylated with tert-butyl bromoacetate to give bis-ester cyclen 1, using established procedures.7 The bridging unit 2 was prepared by condensing two equivalents of chloroacetyl chloride with the corresponding bis-amine derivative8 (see ESI†). Reaction of the 1,7-DO2A diester 1 and the bis-amide compound 2 in acetonitrile under basic conditions afforded macrocycle 3, which was isolated in 70% yield after purification by recrystallization from diethyl ether. Cleavage of the tert-butyl ester groups in 3 followed by complexation of the resulting product with either lutetium or europium trifluoromethanesulfonate salts in methanol, produced the lanthanide complexes 4a and 4b in a quantitative yield. The condensation reaction between 3,5-bis-chlorocarbonyl pyridine and two equivalents of 3-bromopropylamine hydrobromide in dry CH2Cl2 in the presence of triethylamine gave the bis-bromo amide pyridine derivative 5 in 50% yield. Azide nucleophilic substitution followed by oxidation using oxone afforded the bis-azide pyridine N-oxide compound 6 in 80% yield.
The synthesis of the target lanthanide [2]rotaxanes was achieved by a copper(I) catalysed cycloaddition azide–alkyne (CuAAC) click stoppering reaction as shown in Scheme 3. A solution of the appropriate lanthanide macrocyclic complex (4a and 4b), bis-azide pyridine N-oxide axle precursor 6 and two equivalents of alkyne-functionalised terphenyl stopper 7 in dichloromethane in the presence of a catalytic amount of Cu(CH3CN)4PF6 was stirred at room temperature for 48 hours. After purification using size exclusion chromatography, the rotaxanes 8a and 8b were isolated in 20% yield, and were characterized by high-resolution electrospray mass spectrometry (Fig. 1) and 1H NMR.9
![Synthesis of lanthanide [2]rotaxanes 8a and 8b. TBTA = (tris-[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine).](/image/article/2013/CC/c3cc45404e/c3cc45404e-s3.gif) | ||
| Scheme 3 Synthesis of lanthanide [2]rotaxanes 8a and 8b. TBTA = (tris-[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine). | ||
![High resolution electrospray ionization mass spectrum of [2]rotaxane 8b (top) with theoretical isotope model for [M − TfO + Na]2+ (bottom).](/image/article/2013/CC/c3cc45404e/c3cc45404e-f1.gif) | ||
| Fig. 1 High resolution electrospray ionization mass spectrum of [2]rotaxane 8b (top) with theoretical isotope model for [M − TfO + Na]2+ (bottom). | ||
A comparison of the 1H NMR spectra of the [2]rotaxane 8a, macrocycle 4a and stoppered axle is shown in Fig. 2. The upfield shifts of the macrocycle hydroquinone protons observed with 8a are diagnostic of aromatic donor–acceptor stacking interactions between the electron rich hydroquinone groups of the macrocycle and the electron deficient pyridine N-oxide axle motif. Two-dimensional 1H–1H-ROESY spectroscopy was also used to confirm the interlocked nature of rotaxane 8a with through-space correlations between protons in the respective axle and macrocycle components being identified (see Fig. SI8, ESI†).10 An equimolar solution of cyclen macrocycle 3 and pyridine N-oxide derivative 6 in CD2Cl2 revealed no upfield perturbations of the macrocycle's hydroquinone protons which suggests the contribution of aromatic donor–acceptor interactions to the overall mechanical bond formation process is minimal and highlights the crucial templating role of the lanthanide cation.11
![Partial 1H NMR spectra in CDCl3–CD3OD 1 : 1 at 293 K of (a) macrocycle 4a, (b) [2]rotaxane 8a, and (c) axle. For atom labels see Scheme 3.](/image/article/2013/CC/c3cc45404e/c3cc45404e-f2.gif) | ||
Fig. 2 Partial 1H NMR spectra in CDCl3–CD3OD 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 293 K of (a) macrocycle 4a, (b) [2]rotaxane 8a, and (c) axle. For atom labels see Scheme 3. 1 at 293 K of (a) macrocycle 4a, (b) [2]rotaxane 8a, and (c) axle. For atom labels see Scheme 3. | ||
The luminescence from the europium centre was also used to probe the structure of macrocycle 4b and rotaxane 8b in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2
1 CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH and in 1
CH3OH and in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD2Cl2
1 CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD. In the former solvent mixture, the observed luminescence lifetime of 8b was found to be 0.94 ms, while in deuterated media the luminescence lifetime increased to 1.18 ms. Understanding solvation of even simple systems in binary solvent mixtures is not straightforward, but given the residual charge on the lanthanide centre, it is reasonable to assume that any inner sphere solvent at the europium ion will be comprised of methanol rather than CH2Cl2 molecules. As such it is possible to estimate q, the number of inner sphere solvent molecules bound to the lanthanide, from the equation q = 2.4 (τCH3OH−1 − τCD3OD−1 − 0.125 − 0.0375x), where x is the number of exchangeable amide N–H oscillators close to the metal centre.12 In this case, the calculated value of q is 0 if we assume that all four amide N–H oscillators contribute (including the two on the axle), which certainly equates to exclusion of methanol from the inner coordination sphere in 8b, and suggests that the pyridine N-oxide oxygen atom acts as an axial donor to the lanthanide. The luminescence lifetime of 4b in 1
CD3OD. In the former solvent mixture, the observed luminescence lifetime of 8b was found to be 0.94 ms, while in deuterated media the luminescence lifetime increased to 1.18 ms. Understanding solvation of even simple systems in binary solvent mixtures is not straightforward, but given the residual charge on the lanthanide centre, it is reasonable to assume that any inner sphere solvent at the europium ion will be comprised of methanol rather than CH2Cl2 molecules. As such it is possible to estimate q, the number of inner sphere solvent molecules bound to the lanthanide, from the equation q = 2.4 (τCH3OH−1 − τCD3OD−1 − 0.125 − 0.0375x), where x is the number of exchangeable amide N–H oscillators close to the metal centre.12 In this case, the calculated value of q is 0 if we assume that all four amide N–H oscillators contribute (including the two on the axle), which certainly equates to exclusion of methanol from the inner coordination sphere in 8b, and suggests that the pyridine N-oxide oxygen atom acts as an axial donor to the lanthanide. The luminescence lifetime of 4b in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2
1 CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH is shorter and a biexponential fit gives 0.20 and 0.63 ms, indicating solvation at the europium centre in contrast to 8b.
CH3OH is shorter and a biexponential fit gives 0.20 and 0.63 ms, indicating solvation at the europium centre in contrast to 8b.
In summary, the first lanthanide cation-templated synthesis of an interlocked structure has been demonstrated. A pyridine N-oxide ligand threading component coordinates to a lutetium or europium lanthanide complexed DOTA cyclen motif which is itself incorporated into a macrocycle. The novel lanthanide [2]rotaxanes are prepared through stoppering of the pseudorotaxane assemblies.
F.Z. acknowledges the Ministry of Education of Spain for a Postdoctoral contract (Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional I+D+I 2008–2011). O.A.B. thanks the European Research Council for funding under the European Union's Framework Program (FP7/2007–2013) ERC Advanced Grant Agreement no. 267426. M.J.L. thanks the EPSRC for a DTA studentship.
Notes and references
- (a) E. R. Kay and D. A. Leigh, Pure Appl. Chem., 2008, 80, 17 CrossRef CAS; (b) J. A. Faiz, V. Heitz and J.-P. Sauvage, Chem. Soc. Rev., 2009, 38, 422 RSC; (c) L. Fang, M. A. Olson, D. Benítez, E. Tkatchouk, W. A. Goddard III and J. F. Stoddart, Chem. Soc. Rev., 2010, 39, 17 RSC; (d) M. D. Lankshear and P. D. Beer, Acc. Chem. Res., 2007, 40, 657 CrossRef CAS; (e) M. S. Vickers and P. D. Beer, Chem. Soc. Rev., 2007, 36, 211 RSC; (f) S. J. Loeb, Chem. Soc. Rev., 2007, 36, 226–235 RSC.
- (a) G. T. Spence and P. D. Beer, Acc. Chem. Res., 2013, 46(2), 571–586 CrossRef CAS; (b) M. J. Chmielewski, J. J. Davis and P. D. Beer, Org. Biomol. Chem., 2009, 7, 415–424 RSC.
- (a) J. P. Sauvage, Acc. Chem. Res., 1990, 23, 319 CrossRef CAS; (b) J. C. Chambron, J. P. Collin, V. Heitz, D. Jouvenot, J. M. Kern, P. Mobian, D. Pomeranc and J. P. Sauvage, Eur. J. Org. Chem., 2004, 1627 CrossRef CAS; (c) J. F. Stoddart, Templates in Chemistry II, Springer, Berlin/Heidelberg, 2005, pp. 227–240 Search PubMed; (d) J. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh and R. T. McBurney, Angew. Chem., Int. Ed., 2011, 50, 9260–9327 CrossRef CAS; (e) J. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530–1541 RSC.
- We have recently reported lanthanide appended rotaxanes, prepared by post-rotaxane formation. See C. Allain, P. D. Beer, S. Faulkner, M. W. Jones, A. M. Kenwright, N. L. Kilah, R. C. Knighton, T. J. Sorensen and M. Tropiano, Chem. Sci., 2013, 4, 489–493 RSC . Hoffart and Loeb have used pyridine N-oxide at the terminus of rotaxanes for the construction of MOFs. See D. J. Hoffart and S. J. Loeb, Angew. Chem., Int. Ed., 2005, 44, 901–904 CrossRef CAS.
- (a) S. Faulkner, L. S. Natrajan, W. S. Perry and D. Sykes, Dalton Trans., 2009, 3890–3899 RSC; (b) C. Allain and S. Faulkner, Future Med. Chem., 2010, 2, 339–350 CrossRef CAS.
- L. M. Hancock and P. D. Beer, Chem. Commun., 2011, 47, 6012–6014 RSC.
- (a) L. M. De León-Rodríguez, Z. Kovacs, A. C. Esqueda-Oliva and A. D. Miranda-Olvera, Tetrahedron Lett., 2006, 47, 6937–6940 CrossRef; (b) T. Hirayama, M. Taki, A. Kodan, H. Kato and Y. Yamamoto, Chem. Commun., 2009, 3196–3198 RSC.
- L. M. Hancock and P. D. Beer, Chem.–Eur. J., 2009, 15, 42–44 CrossRef CAS.
- The by-products from the reaction were non-interlocked macrocycle and axle.
- Addition of 1 equivalent of axle to macrocycle 4a, in 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD2Cl2–CD3OD, revealed no perturbation of the hydroquinone protons in the 1H NMR spectrum. This further confirms that the upfield shifted hydroquinones observed in rotaxane 8a are due to the interlocked nature of the axle and macrocycle components, and demonstrates that the stopper components prevent the macrocycle threading–dethreading. This rules out the possibility of the axle perching on the outside of the macrocycle, and confirms that the rotaxane is mechanically interlocked (see ESI†).
1 CD2Cl2–CD3OD, revealed no perturbation of the hydroquinone protons in the 1H NMR spectrum. This further confirms that the upfield shifted hydroquinones observed in rotaxane 8a are due to the interlocked nature of the axle and macrocycle components, and demonstrates that the stopper components prevent the macrocycle threading–dethreading. This rules out the possibility of the axle perching on the outside of the macrocycle, and confirms that the rotaxane is mechanically interlocked (see ESI†). - The copper(I) catalysed (CuAAC) stoppering rotaxane synthesis reaction cannot be undertaken in the absence of the lanthanide cation template because of the propensity of the DOTA ligand to complex copper.
- (a) A. Beeby, I. M. Clarkson, R. S. Dickens, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. G. Williams and M. Woods, J. Chem. Soc., Perkin Trans. 2, 1999, 493 RSC; (b) S. Faulkner, S. J. A. Pope and B. P. Burton-Pye, Appl. Spectrosc. Rev., 2005, 40, 1–31 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for synthetic and spectroscopic procedures, and additional characterisation. See DOI: 10.1039/c3cc45404e |
| This journal is © The Royal Society of Chemistry 2013 |
