Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid and efficient synthesis of [11C]ureas via the incorporation of [11C]CO2 into aliphatic and aromatic amines†
Abdul Karim
Haji Dheere
,
Nadiya
Yusuf
and
Antony
Gee
*
Division of Imaging Sciences and Biomedical Engineering, King's College London, UK SE1 7EH. E-mail: antony.gee@kcl.ac.uk; Fax: +44 (0)20 718 85442; Tel: +44 (0)20 718 88366
First published on 31st July 2013
Abstract
A rapid urea radiolabelling methodology has been developed. [11C]CO2 was activated by 1,8-diazabicycloundec-7-ene (DBU) in the presence of aliphatic and aromatic amines and reacted with Mitsunobu reagents to produce asymmetric 11C radiolabelled ureas in high radiochemical yields.
Positron emission tomography (PET) is a non-invasive molecular imaging technique that is used for medical diagnosis, drug development, and the understanding of normo- and pathophysiology.1 Carbon-11 (t1/2 = 20.4 min) is a commonly used radio-isotope for PET imaging, the ubiquity of carbon in all naturally occurring organic compounds making it an attractive radio-isotope for molecular imaging. Substituting carbon-12 (12C) in biologically active molecules with radioactive 11C has no effect on the chemistry or the biological activity of the molecule.2 Cyclotron-produced 11C is commonly prepared in the form of [11C]carbon dioxide ([11C]CO2) by the 14N(p,α)11C nuclear reaction. Due to its poor reactivity, [11C]CO2 is typically converted into more reactive synthons such as [11C]methyl iodide or triflate and subsequently used to radiolabel molecules of biological interest.3 Although these labelling synthons are useful, not all target molecules are accessible by these synthons and their preparation takes several minutes with a concomitant decrease in 11C radioactivity due to decay. The development of methods to efficiently label compounds directly with [11C]CO2 is therefore of significant interest.
To overcome the low reactivity of CO2, bases such as 1,8-diazabicycloundec-7-ene (DBU) or 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP) have recently been utilised as CO2 activating agents in the synthesis of 11C-labelled organic molecules.4,5 These methods, however, produce very poor yields for unreactive aromatic amines, or the reactions are limited to a specific product.6
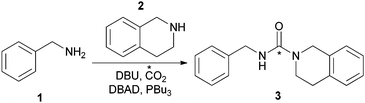
We report herein a rapid [11C]CO2 radiolabelling methodology which overcomes these limitations. DBU was used to trap the cyclotron-produced [11C]CO2 which was subsequently reacted with aliphatic, benzylic and aromatic amines (Scheme 1) to synthesise [11C]ureas in a highly efficient manner.
![Synthesis of [11C]urea with [11C]CO2.](/image/article/2013/CC/c3cc44046j/c3cc44046j-s1.gif) | ||
| Scheme 1 Synthesis of [11C]urea with [11C]CO2. | ||
Ureas are found in a plethora of biologically active molecules as has been extensively reported in the literature.7 The method reported herein provides a methodology to label this class of compounds with carbon-11.
Model reactions were initially conducted using nonradioactive CO2.8 Compound 3 was subsequently chosen as the initial model reaction for optimisation with [11C] CO2.
[11C]CO2 from the cyclotron target was bubbled in a stream of helium gas at a flow rate of 1.4 ml min−1 post target depressurisation directly into a solution containing a primary amine, a secondary amine and DBU in acetonitrile for one minute. The solution was stirred for one minute prior to the addition of Mitsunobu reagents di-tert-butyl azodicarboxylate (DBAD) and tributylphosphine (PBu3).
Initially, experiments were performed in a number of different solvents at 40 °C (Table 1, entries 1–3) with the aim of identifying the best solvent for the reaction. Acetonitrile trapped cyclotron-produced [11C]CO2 very efficiently when bubbled directly into the reaction mixture (>95%), while DMSO and DMF were slightly less efficient (80–90%).
|
|
||||
|---|---|---|---|---|
| Entrya | Solvent | T (°C) | Time (min) | RCYb (%) |
| a Reaction conditions: primary amine (18.3 μmol), secondary amine (27.5 μmol), DBU (0.8 μmol), Mitsunobu reagents (36.6 μmol) in 400 μmol acetonitrile. n = 3. b Determined by radio-HPLC. c Reduced concentration.9 | ||||
| 1 | MeCN | 40 | 5 | 46 ± 7 |
| 2 | DMF | 40 | 5 | 13 ± 3 |
| 3 | DMSO | 40 | 5 | 18 ± 6 |
| 4c | MeCN | 25 | 5 | 0 |
| 5 | MeCN | 25 | 5 | 8 ± 1 |
| 6 | MeCN | 60 | 5 | 26 ± 12 |
| 7 | MeCN | 50 | 5 | 95 ± 3 |
| 8 | MeCN | 50 | 1 | 96 ± 2 |
Moderate radiochemical yields (46%) of the desired [11C]ureas were observed using acetonitrile as a solvent while DMF and DMSO gave yields of 13% and 18% respectively, despite good [11C]CO2 trapping (Table 1). Acetonitrile was therefore selected as the solvent of choice for subsequent reactions.
RCY was determined by radio-HPLC and defined as the amount of labelled [11C]urea as a percentage of the cyclotron-produced [11C]CO2 trapped in solution obtained directly from the cyclotron and corrected for radioactive decay.
When the reactions were carried out at lower reagent concentrations (Table 1, entry 4), no [11C]radiolabelled product was observed despite efficient [11C]CO2 trapping.9
The temperature dependency of the reaction was subsequently examined. Loss of [11C]CO2 from the reaction vial was observed when the reactions were performed at 60 °C. Reactions at 50 °C avoided these losses and resulted in over 95% incorporation of the [11C]CO2 into the target radiolabelled molecules (Table 1, entry 7). Reducing the reaction time from 5 to 1 minute still resulted in a RCY of 96% (Table 1, entry 8 and Fig. 1).
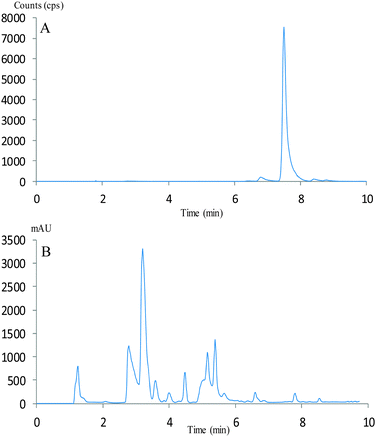 | ||
| Fig. 1 HPLC chromotogram of the crude radiolabelled product (Table 1, entry 8). (A) Radioactivity (counts per second) target compound 3 at Rt 7.30 min. (B) UV absorption (254 nm) of compound 1 at Rt 3.25 min, compound 2Rt 3.45 min and by-products at 5.00 min, 5.30 min and 5.50 min. | ||
The conditions for the model reaction were subsequently applied to the radiosynthesis of various asymmetric ureas using a range of aliphatic, benzylic and aromatic amines (Table 2).
| Entrya | Product | RCYb (%) |
|---|---|---|
| a n = 3. b Determined by radio-HPLC. Reaction conditions: [11C]CO2, primary amine (18.3 μmol), secondary amine (27.5 μmol), DBU (0.8 μmol) in 400 μmol acetonitrile heated at 50 °C for 1 min. Mitsunobu reagents (36.6 μmol) added and stirred for 1 min. | ||
| 1 |
|
74 ± 9 |
| 2 |
|
94 ± 2 |
| 3 |
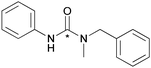
|
69 ± 6 |
| 4 |
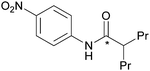
|
85 ± 6 |
| 5 |
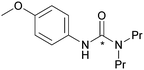
|
83 ± 5 |
| 6 |
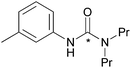
|
80 ± 10 |
| 7 |
|
19 ± 15 |
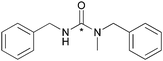
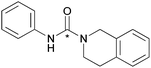
The reactions between benzylic primary amines and the secondary amine, tetrahydroisoquinoline (Table 1, entry 8) resulted in high RCY while the reaction with N-methylbenzylamine produced slightly lower yields of the [11C]urea (Table 2, entry 1). The high yields for tetrahydroisoquinoline can be explained by the rigidity of the molecule, having a locked planer confirmation and less steric hindrance.
Interestingly, RCYs of similar magnitudes were observed when a less reactive aromatic primary amine was used in place of a benzylic amine to form the target radiolabelled molecules (Table 2, entries 2 and 3).
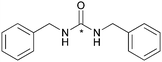
The effect of functional groups on aromatic amines was also studied. Reactions with the electron rich aromatic amines m-toluidine, and p-anisidine resulted in over 80% RCY (Table 2, entries 5 and 6) and even poor nucleophiles such as 4-nitroaniline reacted efficiently, producing high RCY of 85% (Table 2, entry 4). The reaction favours the formation of asymmetric [11C]ureas despite primary amines being present in excess of [11C]CO2. In the absence of secondary amines, various by-products are observed resulting in reduced RCY (Table 2, entry 7).
In conclusion, a rapid and robust methodology for the radiosynthesis of ureas has been developed. The method incorporates [11C]CO2 directly into aliphatic, benzylic and aromatic amines producing the target radiolabelled ureas in high RCY. Overcoming limitations of previous methods, even poorly reactive aromatic amines gave excellent RCY's of asymmetric [11C]ureas within one minute after the addition of Mitsunobu reagents.
This novel radiolabelling methodology opens up new possibilities for 11C radiolabelling molecules for in vivo molecular imaging applications.
Notes and references
- (a) C. R. Child, S. Kealey, H. Jones, P. W. Miller, A. J. P. White, A. D. Gee and N. J. Long, Dalton Trans., 2011, 40, 6210–6215 RSC; (b) V. J. Cunningham, C. A. Parker, E. A. Rabiner, A. D. Gee and R. N. Gunn, Drug Discovery Today, 2005, 2, 311–315 CrossRef CAS; (c) J. Wang and L. Maurer, Curr. Top. Med. Chem., 2005, 5, 1053–1075 CrossRef CAS; (d) N. Oriuchi, T. Higuchi, T. Ishikita, M. Miyakubo, H. Hanaoka, Y. Iida and K. Endo, Cancer Sci., 2006, 97, 1291–1297 CrossRef CAS.
- (a) P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 47, 8998–9033 CrossRef CAS; (b) F. Lodi, C. Malizia, P. Castellucci, G. Cicoria, S. Fanti and S. Boschi, Nucl. Med. Biol., 2012, 39, 447–460 CrossRef CAS.
- (a) A. A. Wilson, A. Garcia, L. Jin and S. Houle, Nucl. Med. Biol., 2000, 27, 529–532 CrossRef CAS; (b) J. R. Atack, P. Scott-Stevens, J. S. Beech, T. D. Fryer, J. L. Hughes, M. C. Cleij, J.-C. Baron, J. C. Clark, R. J. Hargreaves and F. I. Aigbirhio, J. Pharmacol. Exp. Ther., 2007, 320, 1030–1037 CrossRef CAS; (c) J.-K. Chung, Y. Kim, S.-k. Kim, Y. Lee, S. Paek, J. Yeo, J. Jeong, D. Lee, H. Jung and M. Lee, Eur. J. Nucl. Med., 2002, 29, 176–182 CrossRef CAS; (d) R. Bolton, J. Labelled Compd. Radiopharm., 2001, 44, 701–736 CrossRef CAS.
- (a) J. M. Hooker, A. T. Reibel, S. M. Hill, M. J. Schueller and J. S. Fowler, Angew. Chem., Int. Ed., 2009, 48, 3482–3485 CrossRef CAS; (b) A. A. Wilson, A. Garcia, S. Houle and N. Vasdev, Org. Biomol. Chem., 2010, 8, 428–432 RSC; (c) P. J. Riss, S. Lu, S. Telu, F. I. Aigbirhio and V. W. Pike, Angew. Chem., Int. Ed., 2012, 51, 2698–2702 CrossRef CAS; (d) D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, J. Org. Chem., 2005, 70, 5335–5338 CrossRef CAS; (e) M. L. Gray, K. J. Champagne, D. Fauth, J. P. Baltrus and H. Pennline, Int. J. Greenhouse Gas Control, 2008, 2, 3–8 CrossRef CAS.
- (a) L. D. Field, E. T. Lawrenz, W. J. Shaw and P. Turner, Inorg. Chem., 2000, 39, 5632–5638 CrossRef CAS; (b) M. R. Sievers and P. B. Armentrout, Inorg. Chem., 1999, 38, 397–402 CrossRef CAS; (c) Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Surf. Sci., 1995, 335, 258–263 CrossRef CAS; (d) Y. Gu, F. Shi and Y. Deng, J. Org. Chem., 2003, 69, 391–394 CrossRef; (e) W. McGhee and D. Riley, J. Org. Chem., 1995, 60, 6205–6207 CrossRef CAS.
- (a) E. W. van Tilburg, A. D. Windhorst, M. van der Mey and J. D. M. Herscheid, J. Labelled Compd. Radiopharm., 2006, 49, 321–330 CrossRef CAS; (b) A. A. Wilson, A. Garcia, S. Houle, O. Sadovski and N. Vasdev, Chem.–Eur. J., 2011, 17, 259–264 CrossRef CAS.
- (a) C. P. Decicco, J. L. Seng, K. E. Kennedy, M. B. Covington, P. K. Welch, E. C. Arner, R. L. Magolda and D. J. Nelson, Bioorg. Med. Chem. Lett., 1997, 7, 2331–2336 CrossRef CAS; (b) Q.-Z. Zheng, K. Cheng, X.-M. Zhang, K. Liu, Q.-C. Jiao and H.-L. Zhu, Eur. J. Med. Chem., 2010, 45, 3207–3212 CrossRef CAS; (c) K. Sanphanyaa, S. Wattanapitayakul, O. Prangsaengtongc, M. Joc, K. Koizumi, N. Shibaharac, A. Pripremd, V. Fokine and O. Vajragupta, Bioorg. Med. Chem. Lett., 2012, 22, 3001–3005 CrossRef.
- (a) S. L. Peterson, S. M. Stucka and C. J. Dinsmore, Org. Lett., 2010, 12, 1340–1343 CrossRef CAS; (b) C. J. Dinsmore and S. P. Mercer, Org. Lett., 2004, 6, 2885–2888 CrossRef CAS; (c) K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551–2651 CrossRef CAS.
- Reduced concentration conditions: primary amine (6.1 μmol), secondary amine (9.1 μmol), DBU (0.3 μmol), Mitsunobu reagents (12.2 μmol) and 400 μmol acetonitrile.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc44046j |
| This journal is © The Royal Society of Chemistry 2013 |