Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Probing the reactivity of o-phthalaldehydic acid/methyl ester: synthesis of N-isoindolinones and 3-arylaminophthalides†
Sreeman K.
Mamidyala
* and
Matthew A.
Cooper
*
Institute for Molecular Bioscience, University of Queensland, St Lucia, QLD 4072, Australia. E-mail: s.mamidyala@uq.edu.au; m.cooper@uq.edu.au; Fax: +61 733462101; Tel: +61 733462044
First published on 6th August 2013
Abstract
A new method for the synthesis of N-substituted isoindolinones and 3-arylaminophthalides was developed through aza-Wittig/cyclisation. The reaction of o-phthalaldehydic acid methyl ester with benzylic, aromatic and aliphatic azides gave N-isoindolinones whereas reaction of o-phthalaldehydic acid with the aromatic azides gave 3-arylaminophthalides.
N-Substituted isoindolinone derivatives are heterocyclic compounds with a γ-lactam skeleton that have generated considerable interest due to their varied biological activities; anti-inflammatory,1 anti-bacterial,2 anxiolytic,3 inhibition of protein–protein interactions4 and HIV-reverse transcriptase inhibition.5 Furthermore, they are also known to exhibit fluorescent properties, which renders them useful as chemical probes.6
Synthetic approaches to isoindolinones include condensation reaction of amines with isobenzofuranone,7o-phthalaldehyde,8 2-bromomethylbenzoyl methylester,9 reduction of phthalimides,10 and lactamisation.11 Metal-catalysed approaches include Pd-catalysed C–H activation of N-alkoxybenzamides,12 Pt-catalysed reductive coupling,13 Cu-catalysed multicomponent synthesis14 and o-lithiation/cyclisation.15 Other methods include N-capping of amines,16 electrophilic cyclisation,17 and radical cyclisation.18 We were interested in the substrates o-phthalaldehyde, o-phthalaldehydic acid, o-phthalaldehydic acid methylester derivatives as convenient starting points for a one-pot method. The condensation reaction of o-phthalaldehyde with amines resulted in N-isoindolinones,8a whereas aza-Wittig reaction with azides gave both N-isoindolinones and their corresponding bis-products.19 The reaction of o-phthalaldehydic acid with aromatic amines may proceed via the semi-aminol to give 3-arylaminophthalides,20 whereas under reductive C–N coupling conditions13 gave N-isoindolinones presumably via an intermediate amine. The reaction of immobilized o-phthalaldehydic acid ester with amines at higher temperatures gave 3-hydroxyisoindolinones via semi-aminol, whereas at room temperature N-isoindolinones via imine were afforded.11 Ghosh et al.,21 reported a mild method exploring reductive amination/cyclisation using o-phthalaldehydic acid methyl/thiomether ester derivatives and demonstrated that o-phthalaldehydic acid methyl ester derivative was less reactive. The subtle reactivity difference between the o-phthalaldehyde derivatives bearing aldehyde, acid, and methylester gives a different reaction pathway resulting in either N-isoindolinones or 3-substituted phthalides (Scheme 1).
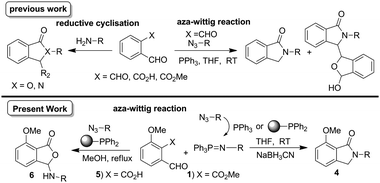 | ||
| Scheme 1 Synthesis of N-isoindolinones and 3-arylaminophthalides. | ||
During our recent investigations, we found that when a Wittig reaction of o-phthalaldehydic acid methylester 1 was carried out, it gave decomposed products, whereas o-phthalaldehydic acid 5 gave the corresponding olefins.22 In this paper, we investigate the aza-Wittig/cyclisation reaction of methylester 1 and o-phthalaldehydic acid 5 resulting in a novel synthesis of N-isoindolinones/3-substituted phthalides respectively (Scheme 1).
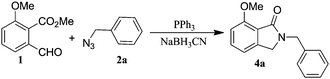
We first investigated the reactivity of o-phthalaldehydic acid methyl ester derivative 1. Substrate 1 and benzyl azide 2a were subjected to aza-Wittig/cyclisation reaction using triphenylphosphine in various solvents. The reaction time was optimised by performing the reactions at different time intervals for aza-Wittig reaction before the addition of reducing agent, NaBH3CN for reductive cyclisation. These reactions revealed that solvent THF was optimal with a 6 h reaction time (Table 1).
|
|
||||
|---|---|---|---|---|
| Entry | Solvent | Temperature | Timeb | Yieldc (%) |
| EDC = ethylene dichloride.a All the reactions were carried with 1 (0.2 mmol), azide 2a (0.3 mmol) and PPh3 (0.4 mmol) in solvent (5.0 mL) and stirred overnight after the addition of NaBH3CN.b Reaction time before addition of NaBH3CN.c Isolated yields. | ||||
| 1 | THF | RT | 3 | 75 |
| 2 | THF | RT | 6 | 90 |
| 3 | THF | RT | 9 | 81 |
| 4 | THF | RT | 12 | 85 |
| 6 | MeCN | RT | 6 | 79 |
| 7 | EDC | RT | 6 | 72 |
| 8 | MeOH | RT | 6 | 0 |
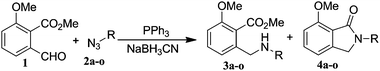
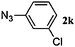
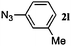
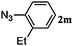
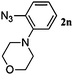
Once the reaction conditions were optimised, we explored various benzylic, aromatic and aliphatic azides. These reactions revealed that: (i) benzylic azides 2a–d and aliphatic azide 2e gave the corresponding N-isoindolinones 4a–e exclusively, (ii) phenyl azide 2f, p-substituted aromatic azides 2g, i gave N-isoindolinones whereas 2h gave both isoindolinone 4h and the amine derivative 3h, (iii) m-substituted aromatic azides 2j–l resulted in the formation of N-isoindolinones 4j–l along with secondary amine derivatives 3j–l, probably due to electronic effects of azides or intermediate secondary amines formed during the reactions, (iv) o-substituted aromatic azides 2m, n and bulky aromatic azide 2o gave secondary amines 3m–o exclusively and the formation of corresponding isoindolinones 4m–o were not observed, this can be attributed to steric factors (Table 2). These electronic or steric effects were not observed in earlier reports where a condensation reaction of aromatic amines with o-phthalaldehyde was performed.8a It was observed that amines 3h, 3j–l and 3o were unstable and converted to their corresponding isoindolinones on standing. To overcome the difficulty in purification due to contamination of triphenylphosphine oxide that was formed during the reactions, we explored the use of polymer-bound triphenylphosphine as an alternate strategy for the synthesis of isoindolinones.
|
|
|||||
|---|---|---|---|---|---|
| Entry | Azides | Timeb (h) | Product ratio | Yieldc (%) | |
| 3 (%) | 4 (%) | ||||
| a All the reactions were stirred at RT for 6 h before the addition of NaBH3CN. b Time required for reductive cyclisation. c Isolated yields. | |||||
| 1 |
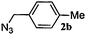
|
12 | 3b (00) | 4b (100) | 72 |
| 2 |
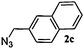
|
12 | 3c (00) | 4c (100) | 55 |
| 3 |
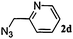
|
12 | 3d (00) | 4d (100) | 86 |
| 4 |

|
12 | 3e (00) | 4e (100) | 69 |
| 5 |

|
24 | 3f (00) | 4f (100) | 95 |
| 6 |
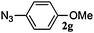
|
48 | 3g (00) | 4g (100) | 94 |
| 7 |
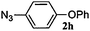
|
48 | 3h (13) | 4h (64) | 77 |
| 8 |
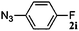
|
48 | 3i (00) | 4i (100) | 80 |
| 9 |
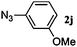
|
48 | 3j (32) | 4j (63) | 95 |
| 10 |
|
48 | 3k (23) | 4k (69) | 92 |
| 11 |
|
48 | 3l (32) | 4l (57) | 89 |
| 12 |
|
48 | 3m (100) | 4m (00) | 75 |
| 13 |
|
48 | 3n (100) | 4n (00) | 55 |
| 14 |
|
48 | 3o (100) | 4o (00) | 42 |
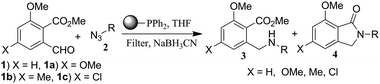
We performed an aza-Wittig reaction of 1 with 2a in THF using polymer-bound triphenylphosphine for 3 h followed by the addition of NaBH3CN and optimised the reaction conditions. An analogous trend was observed for various azides when subjected to aza-Wittig reaction using polymer-bound triphenylphosphine (Table 3). Benzylic 2a, b, aliphatic 2e and p-substituted aromatic azide 2g gave N-isoindolinones exclusively, whereas azide 2h and m-substituted aromatic azides 2j, k gave the corresponding secondary amines. Moreover, these reactions indicated that benzylic azides 2a, b, and aliphatic azide 2e required less time for cyclisation than aromatic azides 2g, h, j, k (Table 3), which might be due to the higher reactivity of secondary amines formed during the reaction. The scope of the reaction was successfully demonstrated by exploring various substituted o-phthalaldehydic derivatives (Table 3, entries 8–10).
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Azides | Timeb (h) | Product ratio | Yielde (%) | |
| 3 (%) | 4 (%) | |||||
| a Conditions: 1 (0.1 mmol), azide 2a (0.2 mmol) and polymer-bound PPh3 (0.2 mmol) in THF (2.5 mL), RT, 3 h, then filter the resin, added NaBH3CN (0.2 mmol). b Reaction time for cyclisation at reflux temperature. c Reaction time for cyclisation at RT. d Reaction performed in MeOH at reflux temperature. e Isolated yields. | ||||||
| 18c | 3a (00) | 4a (100) | 76 | |||
| 1 | 1 | 2a | 3 | 3a (00) | 4a (100) | 80 |
| 3 | 3a (00) | 4a (00) | ||||
| 2 | 1 | 2b | 3 | 3b (00) | 4b (100) | 72 |
| 3 | 1 | 2e | 3 | 3e (00) | 4e (100) | 59 |
| 4 | 1 | 2g | 24 | 3g (00) | 4g (100) | 79 |
| 5 | 1 | 2h | 24 | 3h (100) | 4h (00) | 47 |
| 6 | 1 | 2j | 24 | 3j (100) | 4j (00) | 72 |
| 7 | 1 | 2k | 24 | 3k (100) | 4k (00) | 64 |
| 8 | 1a | 2b | 12 | 3ab (00) | 4ab (100) | 56 |
| 9 | 1b | 2b | 12 | 3bb (00) | 4bb (100) | 60 |
| 10 | 1c | 2b | 12 | 3cb (00) | 4cb (100) | 68 |
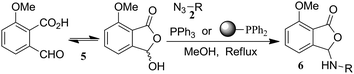
Our success in probing the reactivity of methyl ester 1 by aza-Wittig reaction prompted us to examine the reactivity of o-phthalaldehydic acid derivative 5, which was in equilibrium with its corresponding lactol. Thus, subjecting acid 5 to aza-Wittig reaction with aromatic azides 2f, g, j, and 2m resulted in the isolation of 3-arylaminophthalides (Table 4, entries 3–6). Notably, similar reactions with benzylic azide 2a and aliphatic azide 2e have failed to give the corresponding 3-arylaminophthalides (Table 4, entries 1, 2); consistent with earlier experiments conducted with corresponding amines.20 The structures of the 3-arylaminophthalides were confirmed by comparison of the spectral data with the authentic compounds prepared using a literature procedure.20
|
|
||||
|---|---|---|---|---|
| Entry | Azides | Product | Solvent | Yieldc (%) |
| a Conditions: 5 (0.15 mmol), azide 2a (0.3 mmol) and polymer-bound PPh3 (0.3 mmol), MeOH (5.0 mL), reflux, 5 h. b Reaction using PPh3. c Isolated yields. | ||||
| 1 | 2a | 6a | MeOH | 00 |
| 2 | 2e | 6e | MeOH | 00 |
| MeOHb | 50 | |||
| 3 | 2f | 6f | MeOH | 78 |
| THF | 54 | |||
| 4 | 2g | 6g | MeOH | 66 |
| 5 | 2j | 6j | MeOH | 48 |
| 6 | 2m | 6m | MeOH | 36 |
Our findings indicate that isoindolinones prepared by aza-Wittig reaction using methyl ester 1 was via amine intermediate ‘C’ not the iminium ion ‘D’ (Scheme 2). The formation of 3-arylaminophthalides using o-phthalaldehydic acid 5 could be via intermediate ‘E’. However, it can be argued that it could be formed via aromatic amines generated from the corresponding azides at higher temperatures in protic solvents such as MeOH.23 To probe this issue, reaction of 5 with 2f was conducted in the aprotic solvent, THF, which resulted in isolation of the corresponding 3-arylaminophthalide 6f. This suggests the reaction proceeded via aza-Wittig adduct (Scheme 2).
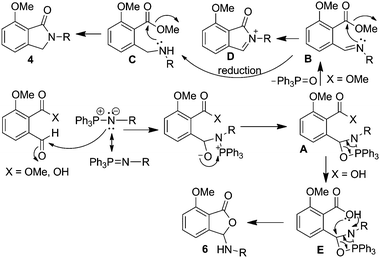 | ||
| Scheme 2 Proposed mechanism for aza-Wittig/cyclisation reaction of o-phthalaldehydic acid/methyl ester derivatives. | ||
In summary, a new entry into the synthesis of N-substituted isoindolinones and 3-arylaminophthalides was developed through aza-Wittig/cyclisation reaction. The reaction of o-phthalaldehydic acid methyl ester with azides gave novel N-isoindolinones and the electronic and steric effects were investigated. Reaction of o-phthalaldehydic acid with aromatic azides gave novel 3-arylaminophthalides, whereas reaction with benzylic and aliphatic azides failed.
We are grateful to National Health & Medical Research Council (NHMRC Australia Fellowship AF511105) for financial support.
Notes and references
- A. L. Ruchelman, H. W. Man, W. Zhang, R. Chen, L. Capone, J. Kang, A. Parton, L. Corral, P. H. Schafer, D. Babusis, M. F. Moghaddam, Y. Tang, M. A. Shirley and G. W. Muller, Bioorg. Med. Chem. Lett., 2013, 23, 360–365 CrossRef CAS.
- J. C. Breytenbach, S. van Dyk, I. van den Heever, S. M. Allin, C. C. Hodkinson, C. J. North and M. I. Page, Bioorg. Med. Chem. Lett., 2000, 10, 1629–1631 CrossRef CAS.
- D. J. M. Mark, H. Norman and G. C. Rigdon, J. Med. Chem., 1996, 39, 149–157 CrossRef.
- I. R. Hardcastle, S. U. Ahmed, H. Atkins, A. H. Calvert, N. J. Curtin, G. Farnie, B. T. Golding, R. J. Griffin, S. Guyenne, C. Hutton, P. Kallblad, S. J. Kemp, M. S. Kitching, D. R. Newell, S. Norbedo, J. S. Northen, R. J. Reid, K. Saravanan, H. M. Willems and J. Lunec, Bioorg. Med. Chem. Lett., 2005, 15, 1515–1520 CrossRef CAS.
- X. Z. Zhao, E. A. Semenova, B. C. Vu, K. Maddali, C. Marchand, S. H. Hughes, Y. Pommier, J. Terrence and R. Burke, J. Med. Chem., 2008, 51, 251–259 CrossRef CAS.
- R. F. Suven Das and A. Pramanik, Org. Lett., 2006, 8, 4263–4266 CrossRef.
- F. M. Rowe, E. Levin, A. C. Burns, J. S. H. Davies and W. Tepper, J. Chem. Soc., 1926, 129, 690 RSC.
- (a) I. Takahashi, T. Kawakami, E. Hirano, H. Yokota and H. Kitajima, Synlett, 1996, 353–355 CrossRef CAS; (b) R. Grigg, H. Q. N. Gunaratne and V. Sridharan, J. Chem. Soc., Chem. Commun., 1985, 1183 RSC.
- H. Wang and A. Ganesan, Tetrahedron Lett., 1998, 39, 9097–9098 CrossRef CAS.
- S. Das, D. Addis, L. R. Knopke, U. Bentrup, K. Junge, A. Bruckner and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 9180–9184 CrossRef CAS.
- K. Knepper, R. E. Ziegert and S. Bräse, Tetrahedron, 2004, 60, 8591–8603 CrossRef CAS.
- (a) J. W. Wrigglesworth, B. Cox, G. C. Lloyd-Jones and K. I. Booker-Milburn, Org. Lett., 2011, 13, 5326–5329 CrossRef CAS; (b) D. D. Li, T. T. Yuan and G. W. Wang, Chem. Commun., 2011, 47, 12789–12791 RSC.
- L. H. L. Shi, J. Wang, X. Cao and H. Gu, Org. Lett., 2012, 14, 1876–1879 CrossRef CAS.
- L. X. Sun, T. Zeng, D. Jiang, L.-Y. Dai and C.-J. Li, Can. J. Chem., 2012, 90, 92–99 CrossRef CAS.
- K. Smith, G. A. El-Hiti and A. S. Hegazy, Chem. Commun., 2010, 46, 2790–2792 RSC.
- D. C. G. D. Augner, N. Slavov, J.-M. Neudorfl and H.-G. Schmalz, Org. Lett., 2011, 13, 5374–5377 CrossRef CAS.
- T. Yao and R. C. Larock, J. Org. Chem., 2005, 70, 1432–1437 CrossRef CAS.
- G. López-Valdez, S. Olguín-Uribe, A. Millan-Ortíz, R. Gamez-Montaño and L. D. Miranda, Tetrahedron, 2011, 67, 2693–2701 CrossRef.
- M. F. T. Aubert and R. Guilar, Can. J. Chem., 1990, 68, 842–851 CrossRef.
- Y. Kubota and T. Tatsuno, Chem. Pharm. Bull., 1971, 19, 1226–1233 CrossRef CAS.
- U. Ghosh, R. Bhattacharyya and A. Keche, Tetrahedron, 2010, 66, 2148–2155 CrossRef CAS.
- S. K. Mamidyala, S. Ramu, J. X. Huang, A. A. Robertson and M. A. Cooper, Bioorg. Med. Chem. Lett., 2013, 23, 1667–1670 CrossRef CAS.
- B. Pal, P. Jaisankar and V. S. Giri, Synth. Commun., 2004, 34, 1317–1323 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc43838d |
| This journal is © The Royal Society of Chemistry 2013 |