 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancing surface reactivity with a noble metal
Hatem
Altass
,
Albert F.
Carley
,
Philip R.
Davies
* and
Robert J.
Davies
Cardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK. E-mail: daviespr@cf.ac.uk; Tel: +44 (0)29 20874072
First published on 30th July 2013
Abstract
Gold, the archetypal noble metal, is usually associated with an inhibition of surface reactivity by site blocking. In this paper however, we show that on Cu(100) surfaces a gold adlayer can actually increase the extent of reaction with the substrate.
The surface structure and topography of gold adsorbed on copper surfaces and of gold–copper alloys has received considerable attention due to the miscibility of the two metals over all concentration ranges and the importance of gold–copper alloys in both the theoretical understanding and practical applications of alloy systems.1–4 Copper has been used to modify the catalytic activity of gold nanoparticles for several oxidation reactions including CO,5 propene6 and benzyl alcohol to yield benzaldehyde.7 Surprisingly, despite the increasing importance of Cu–Au alloys in catalysis there have been no fundamental investigations into chemisorption and reaction at these alloy surfaces. Our aim in this work was to investigate how gold modified the reactivity of copper towards an aggressive molecule such as HCl. We have explored a range of gold concentrations on both Cu(111) and Cu(100) surfaces and these will be described in a more lengthy and detailed publication in the near future; we report here on the dramatic effect of high gold concentrations on the adsorption of HCl at Cu(100) surfaces.
There is a substantial body of literature on the surface chemistry of bimetallics8,9 and specifically the structural and electronic effects2–4 of gold deposited on copper surfaces. There is agreement between the various authors4,10–12 that Au evaporated onto the Cu(100) surface undergoes a place exchange with the Cu atoms in the terraces forming domains of c(2 × 2) Au–Cu alloy. At this stage the Au is largely confined to the uppermost layer. With increasing coverage stress develops in the alloy layer since the Au–Cu lattice parameter is ∼4% larger than that of the copper and this is relieved by the development of nanometer scale defects. At 0.6 monolayers (1 mL = 1.54 × 1015 atoms cm−2) there is evidence for Au in the 2nd and 3rd layers11 but at ∼1 monolayer dealloying occurs with the development of a hexagonal pure gold adlayer and the removal of most of the Au from the underlying layers.
XPS & STM studies of the reaction of HCl with clean Cu(100) surfaces show the chemisorption of Cl adatoms with a limiting coverage of ∼7.1 × 1014 cm−2 reached after ∼200 L and the formation of a c(2 × 2) surface adlayer. During adsorption there is considerable movement of step edges and at low coverages STM resolution is very poor because of the relatively mobile Cl(a) adlayer. The Cl(2p) binding energy of Cl(a) on Cu(100) is at ∼198.0 eV and invariant with coverage. This is consistent with previous studies of low exposures of Cl2 at this surface but unlike HCl, high exposures of Cl2 lead to further adsorption and the formation of a state with a similar binding energy to CuCl at ∼199 eV.13,14
With a noble metal such as gold, adsorption at a Cu(100) surface might have been predicted to result in a simple site blocking model such as that seen for Cu(111)–Au–O2 for example,17 but in the case of HCl this is not the case. The XP spectra in Fig. 1 show that despite the presence of 7.8 × 1014 Au atoms cm−2, slightly in excess of the quantity required for the c(2 × 2) monolayer, reaction with HCl is just as rapid and extensive as in the absence of gold; an exposure of 200 L results in a Cl(a) concentration of 7.4 × 1014 cm−2. In fact, even the presence of a gold concentration well in excess of a monolayer (1.7 × 1015 cm−2), which is known to result in a hexagonal pure Au(111) uppermost adlayer,11 does not stop the HCl reaction. Indeed, it seems to facilitate reaction with an exposure of ∼200 L giving a Cl(a) concentration of 9.9 × 1014, 30% more than the limiting concentration of Cl(a) when the clean surface is exposed to HCl.
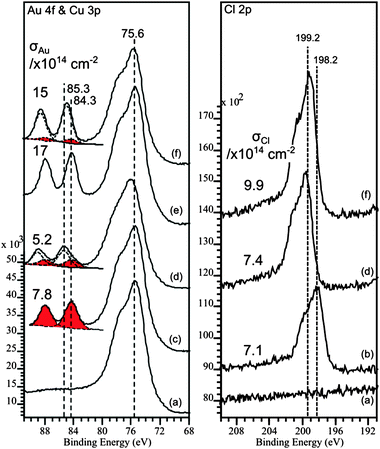 | ||
| Fig. 1 Au(4f) and Cl(2p) XP spectra showing the effect the presence of gold at a Cu(100) surface has on the chemisorption of HCl at room temperature. Surface concentrations of Au and Cl (calculated from the XP peak areas15,16) are indicated on the relevant sets of spectra. (a) Clean surface; (b) Cl(2p) spectrum after exposure of a clean surface in the absence of gold to 230 L HCl. (c) Au(4f) spectrum for Au evaporated onto the clean Cu(100) surface, (d) Au(4f) and Cl(2p) spectra after exposure of surface in (c) to 200 L HCl; (e) Au(4f) spectrum for a higher concentration of Au at the clean Cu(100) surface; (f) Au(4f) and Cl(2p) spectra after exposure of surface in (e) to 200 L HCl. | ||
Interestingly, in both cases the Cl(2p) binding energy is ∼199 eV, 1 eV higher than Cl on clean Cu(100) and 1.7 eV higher than that expected for Cl(a) on gold.13 It is also noteworthy that the apparent Au concentration is significantly lower after exposure to the HCl and a second Au state is now evident from the broadening of the 4f peaks. Curve fitting of the spectra in Fig. 1(a) show the two components: one with a Au 4f7/2 peak at the same binding energy of 84.3 eV as the clean alloy the other at a much higher binding energy of 85.2 eV. The higher binding energy species cannot be assigned to a gold chloride since the Cl(2p) binding energy is considerably higher than that associated with Cl on Au surfaces.13 However, the relatively high binding energy may be due to the formation of smaller pure gold nanoparticles after reaction with HCl has removed all the copper, the Au 4f binding energy having been shown to be sensitive to particle size and alloy composition.3,18
STM images were recorded during the two experiments described above. In both cases, before exposure to HCl the surfaces consist of small terraces separated by steps of ∼0.19 nm most containing rounded islands of the same height (Fig. 2(a) and (e)). It is known that for coverages up to approximately 7 × 1014 cm−2 the majority of Au is adsorbed into the uppermost copper layer forming a c(2 × 2) ordered alloy, but for coverages in excess of a monolayer, the gold de-alloys from the copper to form an upper layer consisting of a pure hexagonal Au(111) adlayer.19 During the exposure to HCl there was considerable movement of both step edges and islands for both concentrations of gold, but very different STM images were obtained for the two gold concentrations after the ∼200 L dose of HCl. In the case of the lower gold concentration, areas of terrace were covered in small domains (∼25 nm2) of a regular square structure with a lattice parameter of ∼0.51 nm.
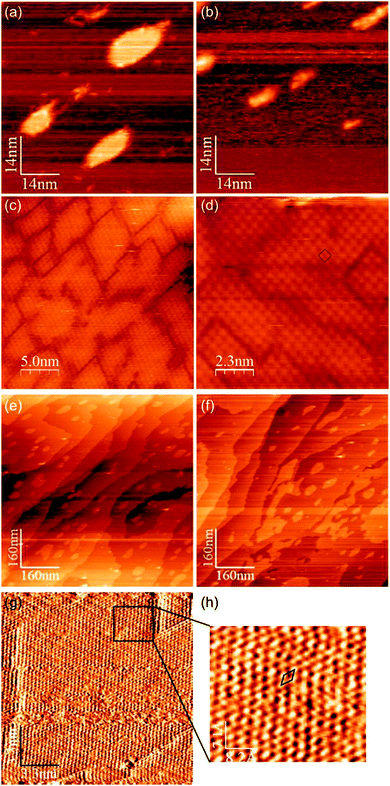 | ||
| Fig. 2 STM images showing topographical effects of HCl adsorption at Cu(100) surfaces with two different concentrations of Au adsorbed: σAu = 7.8 × 1014 cm−2 images (a)–(d). (a) Cu–Au alloy islands before HCl exposure, (b) during exposure to HCl at 1 × 10−8 mbar, the islands have disintegrated as Cu is extracted by reaction with HCl. (c) and (d) Images recorded after ∼200 L Cl showing the square lattice consistent with a c(2 × 2) Cl(a) adlayer. σAu = 1.7 × 1015 cm−2, images (e)–(h). (e) and (f) Before and after exposure to HCl showing large scale island sintering and step movement. (g) and (h) Close up images showing atomic resolution of the hexagonal lattice consistent with a Au(111) surface. | ||
This is consistent with the formation of a c(2 × 2) Cl adlayer and in light of the marked decrease in the Au 4f signal (Fig. 1(c) and (d)) suggests the formation of a CuCl adlayer over the Cu–Au alloy (Fig. 3(a)). The high Au coverage surface gives a slightly different result; again there appears to have been significant step edge movement and sintering of the islands on the terraces but also, where atomic resolution can be obtained after reaction a hexagonal lattice is observed with a lattice parameter of 0.3 nm consistent with the Au(111) surface present before HCl exposure. The very high Cl concentration and Cl(2p) binding energy indicate the formation of a CuCl state and again a reduction in Au(4f) intensity suggests a buried layer of Cu–Au alloy, Fig. 3(b).
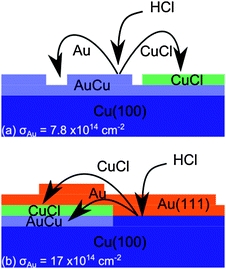 | ||
| Fig. 3 Models for the adsorption of HCl on Cu(100) surfaces in the presence of (a) monolayer and (b) multi-monolayer coverages of Au. In both cases the XP spectra (Fig. 1) suggest a CuCl state and a buried Cu–Au alloy. | ||
Our models for the reaction of HCl with the Cu(100)–Au surfaces with different Au coverages are summarised in Fig. 3; in both cases HCl reacts with the copper giving rise to a copper chloride that develops on top of the Au–Cu alloy. However, what we wish to draw attention to in this experiment is the unusual effect of gold acting as a facilitator of the reaction of HCl with the copper. Furthermore it is evident from the high Cl(2p) binding energy and the very high Cl concentrations achieved that the chloride phase created is closer to a CuCl or CuCl2 state than a simple chemisorbed Cl adlayer. Previously this state has only been obtained with extensive exposure to Cl2 or, from HCl in the presence of surface oxygen. The latter has been attributed17 to the thermodynamic advantage provided when the HCl reacts with a copper surface already reconstructed by the oxygen. It appears that gold may be able to effect the same facile reaction.
The research was supported by EPSRC grant EP/I038748/1 and HA was supported by a grant from the Government of Saudi Arabia.
Notes and references
- C. L. Bracey, P. R. Ellis and G. J. Hutchings, Chem. Soc. Rev., 2009, 38, 2231 RSC.
- M. Kuhn and T. K. Sham, Phys. Rev. B, 1994, 49, 1647–1661 CrossRef CAS.
- T. K. Sham, A. Hiraya and M. Watanabe, Phys. Rev. B, 1997, 55, 7585–7592 CrossRef CAS.
- D. D. Chambliss and S. Chiang, Surf. Sci., 1992, 264, L187–L192 CrossRef CAS.
- X. Liu, A. Wang, T. Zhang, D.-S. Su and C.-Y. Mou, Catal. Today, 2011, 160, 103–108 CrossRef CAS.
- C. L. Bracey, A. F. Carley, J. K. Edwards, P. R. Ellis and G. J. Hutchings, Catal. Sci. Technol., 2011, 1, 76 CAS.
- C. Della Pina, E. Falletta and M. Rossi, J. Catal., 2008, 260, 384–386 CrossRef CAS.
- V. Ponec, Surf. Sci., 1979, 80, 352–366 CrossRef CAS.
- V. Ponec, Appl. Catal., A, 2001, 222, 31–45 CrossRef CAS.
- P. W. Palmberg and T. N. Rhodin, J. Chem. Phys., 1968, 49, 134–146 CrossRef CAS.
- D. Naumović, A. Stuck, T. Greber, J. Osterwalder and L. Schlapbach, Surf. Sci., 1992, 269–270, 719–723 CrossRef.
- L. H. Zhou, C. S. Tian, N. Lei, G. S. Dong and X. F. Jin, J. Phys.: Condens. Matter, 2010, 22, 395007 CrossRef CAS.
- K. Kishi and S. Ikeda, J. Phys. Chem., 1974, 78, 107–112 CrossRef CAS.
- W. Sesselmann and T. J. Chuang, Surf. Sci., 1986, 176, 32–66 CrossRef CAS.
- A. F. Carley and M. W. Roberts, Proc. R. Soc. London, Ser. A, 1978, 363, 403–424 CrossRef CAS.
- A. F. Carley, P. R. Davies, R. V. Jones, K. R. Harikumar, G. U. Kulkarni and M. W. Roberts, Surf. Sci., 2000, 447, 39–50 CrossRef CAS.
- H. Altass, A. F. Carley, P. R. Davies and R. J. Davies, 2013, in preparation.
- C. N. R. Rao, V. Vijayakrishnan, H. N. Aiyer, G. U. Kulkarni and G. N. Subbanna, J. Phys. Chem., 1993, 97, 11157–11160 CrossRef CAS.
- D. Chambliss, R. Wilson and S. Chiang, J. Vac. Sci. Technol., A, 1992, 10, 1993–1998 CAS.
| This journal is © The Royal Society of Chemistry 2013 |
