 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
‘Sergeants-and-Corporals’ principle in chiral induction at an interface†
Iris
Destoop‡
a,
Hong
Xu‡
a,
Cristina
Oliveras-González
b,
Elke
Ghijsens
a,
David B.
Amabilino
*b and
Steven
De Feyter
*a
aKU Leuven, Celestijnenlaan 200F, 3001 Heverlee, Belgium. E-mail: steven.defeyter@chem.kuleuven.be; Fax: +32 1632 7990; Tel: +32 1632 7921
bInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus Universitari, 08193 Bellaterra, Catalonia, Spain. E-mail: amabilino@icmab.es; Fax: +34 935 805 729; Tel: +34 935 801 853
First published on 13th May 2013
Abstract
Self-assembly of an achiral porphyrin at the interface between a chiral solvent and an atomically flat substrate renders the monolayer chiral, and a non-racemic solvent can even overrule the intrinsic expression of chirality in the self-assembly of chiral molecules.
Chiral expression at surfaces has attracted increasing attention in recent years, partially because of its importance in optical resolution1 and enantioselective heterogeneous catalysis.2 Self-assembly of achiral and chiral organic building blocks is one of the simplest ways to construct surfaces with two-dimensional (2D) supramolecular chirality.3–5 In general, enantiopure molecules form chiral layers: mirror image patterns are absent and hence the surface exhibits global chirality. Systems based on achiral building blocks form both mirror image patterns with an equal probability and the surfaces are therefore globally achiral.6
Global chirality can still be obtained for achiral building blocks by using a chiral input. Addition of small amounts of chiral auxiliaries can force the achiral molecules to form domains with a particular handedness (the Sergeant-and-Soldiers effect).7,8 Such effects have been amply demonstrated for fibre-like supramolecular structures in solution.9 In this case the chirality of the auxiliary determines the absolute handedness of the supramolecular string. Recently it was also shown that self-assembly of achiral molecules from chiral solvents can create chiral monolayers.10 From these observations the question arises whether chiral solvents can act as “Corporals” and overrule the intrinsic chirality of the molecular building blocks (the “Sergeants”). In this communication we address this question.
The self-assembly of porphyrins 1–4 (Fig. 1) at the interface between a liquid and graphite leads to the formation of quasi-isostructural domains existing in regular rows of molecules.11 This family of molecules comprises (i) an achiral porphyrin (1), (ii) a chiral porphyrin substituted with one chiral side-chain (2), (iii) a chiral porphyrin with two chiral side-chains at the 5- and 10-positions (S,S-3) and (iv) a chiral porphyrin with two chiral side-chains at 5- and 15-positions (S,S-4).
 | ||
| Fig. 1 Molecular structures of the porphyrin derivatives. | ||
The monolayer handedness of these molecules was previously investigated at the achiral 1-heptanol/graphite interface and is expressed at the level of rotation (φ) of the molecular row with respect to the underlying substrate.11 Either a clockwise (CW) or an anticlockwise (CCW) rotation should be exerted in order to define the relationship between a reference axis of the surface and the molecular row (see for instance Fig. 2 for the principle). Two packing arrangements with opposite handedness are formed for 1: as expected for an achiral molecule both CW and CCW domains with an angle φ = +6 ± 4° and φ = −8 ± 2°, respectively, are present equally. For all the derivatives with one or more chiral side chains mirror image patterns are essentially absent§ and the rotation with respect to the substrate (the monolayer chirality descriptor) is determined by the nature of the stereogenic centre in the porphyrins: those with S-stereogenic centres are always rotated CCW and those with R-stereogenic centres are always rotated CW with respect to the reference axis. This result indicates that the stereogenic centres affect the self-assembly process.
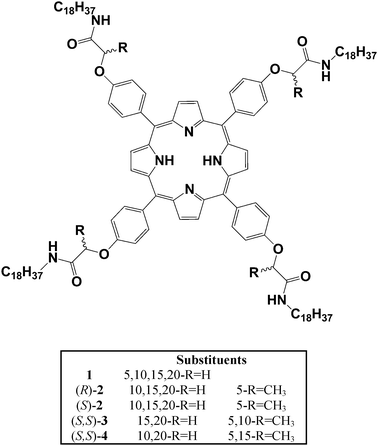
 | ||
| Fig. 2 STM images of the achiral porphyrin 1 physisorbed at the (A) (S)-2-octanol/HOPG interface or (B) (R)-2-octanol/HOPG interface. The black solid lines indicate the direction of the main symmetry axes of the underlying graphite. The dashed red lines indicate the reference axis of graphite 〈−1 1 0 0〉. The white lines indicate the lamellar direction. Unit cells are indicated in green. φ is the angle between the reference axis and the unit cell vector a. The yellow arrows display the rotation direction. Tunnelling parameters are Iset = 600 pA and Vbias = −1 mV. | ||
So far the self-assembly of porphyrins 1–4 has only been observed in the achiral protic solvent 1-heptanol. From molecular dynamics (MD) simulations Linares et al. concluded that the amide groups adopt conformations that allow hydrogen bonding with solvent molecules, leading to stabilization of the porphyrin monolayers.12 For this reason the chiral hydrogen bonding solvents (S)- and (R)-2-octanol were chosen to probe the effect of chiral solvent on the monolayer structure. The porphyrins were dissolved in these solvents and diluted to a concentration of 0.1 mg mL−1, then drop-cast onto the basal plane of freshly-cleaved highly oriented pyrolytic graphite (HOPG) and examined by scanning tunneling microscopy (STM) under ambient conditions in the solvent. First, we established whether the solvent is able to affect the self-assembly of achiral 1. Indeed, CW and CCW domains are not equally expressed anymore when 1 is dissolved in enantiopure 2-octanol. The majority of the domains (92%) showed a CW rotation (+6 ± 3°) with respect to the reference axis in (S)-2-octanol (Table 1). Only 8% of domains showed the CCW structure, characterized by φ = −5 ± 3°. Repeating this experiment in (R)-2-octanol revealed the opposite effect: 80% of the domains were CCW (−9 ± 5°) (Fig. 2B). The values for the unit cells of induced domains are a = 1.9 ± 0.1 nm, b = 4.1 ± 0.2 nm, γ = 80 ± 4° and a = 1.9 ± 0.2 nm, b = 3.9 ± 0.1 nm, γ = 83 ± 4° for (S)- and (R)-2-octanol, respectively, and these are identical to those observed in 1-heptanol.
| 1-Heptanol | (S)-2-Octanol | (R)-2-Octanol | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | φ (deg) | N | a (nm) | b (nm) | γ (deg) | φ (deg) | N | a (nm) | b (nm) | γ (deg) | φ (deg) | |
| a As some molecules form two types of domains with φ > 0 and φ<0, values for these domains are treated separately. | ||||||||||||
| 1 | 10 | +5 ± 4 | 34 | 1.9 ± 0.1 | 4.1 ± 0.2 | 80 ± 4 | +6 ± 3 | 8 | 1.9 ± 0.1 | 3.9 ± 0.1 | 83 ± 4 | +4 ± 2 |
| 10 | −10 ± 4 | 3 | 1.8 ± 0.1 | 3.9 ± 0.2 | 79 ± 3 | −5 ± 3 | 32 | 1.9 ± 0.2 | 3.9 ± 0.1 | 83 ± 4 | −9 ± 5 | |
| R-2 | 30 | +7 ± 4 | 17 | 1.8 ± 0.2 | 3.8 ± 0.1 | 81 ± 5 | +10 ± 5 | 32 | 1.7 ± 0.2 | 4.0 ± 0.2 | 79 ± 5 | +7 ± 3 |
| 1 | −15 | 0 | — | — | — | — | 7 | 1.8 ± 0.1 | 4.0 ± 0.2 | 83 ± 2 | −6 ± 5 | |
| S-2 | 17 | −8 ± 3 | 31 | 1.8 ± 0.1 | 4.0 ± 0.2 | 81 ± 4 | −6 ± 3 | 17 | 1.8 ± 0.1 | 4.0 ± 0.1 | 82 ± 5 | −6 ± 5 |
| 2 | +0.4 ± 1 | 7 | 1.8 ± 0.1 | 4.0 ± 0.1 | 78 ± 2 | +6 ± 2 | 1 | 1.8 | 4.0 | 82 | +3 | |
| S,S-3 | 9 | −8 ± 2 | 26 | 1.9 ± 0.1 | 4.1 ± 0.3 | 82 ± 4 | −10 ± 3 | 15 | 1.9 ± 0.1 | 4.0 ± 0.2 | 83 ± 4 | −7 ± 3 |
| 0 | — | 1 | 1.8 | 3.7 | 83 | +7 | 0 | — | — | — | — | |
| S,S-4 | 10 | −8 ± 2 | 23 | 1.8 ± 0.1 | 4.0 ± 0.2 | 80 ± 4 | −7 ± 4 | 9 | 1.9 ± 0.1 | 4.1 ± 0.1 | 80 ± 4 | −6 ± 3 |
The self-assembly of the achiral derivative is clearly influenced by the chiral medium, but what happens when a chiral porphyrin is adsorbed from a chiral solvent? When (S)-2-octanol was used as solvent, for (R)-2, with one stereogenic centre, only CW domains are formed, identical to the situation in 1-heptanol.
In contrast, in the presence of enantiomeric (R)-2-octanol, the surface is not only covered with CW domains (+7 ± 3°), but also with CCW domains (−6 ± 5°) (∼18% of 39 domains, Fig. 3A). The unit cell parameters of the layer are otherwise identical (Table 1).
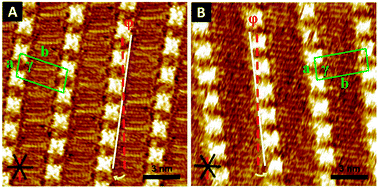
 | ||
| Fig. 3 STM images of chiral porphyrin (A) (R)-2 physisorbed at the (R)-2-octanol/HOPG interface and (B) (S)-2 physisorbed at the (S)-2-octanol/HOPG interface. The black solid lines indicate the direction of the main symmetry axes of the underlying graphite. The dashed red lines indicate the reference axis of graphite 〈−1 1 0 0〉. The white lines indicate the lamellar direction. Unit cells are indicated in green. φ is the angle between the reference axis and the unit cell vector a. The yellow arrows display the rotation direction. (C) Large scale STM image of (S)-2 at the (S)-2-octanol/HOPG interface. Domain boundaries are indicated by dashed yellow lines. Both CW and CCW domains are present. Tunnelling parameters are Iset = 280 pA and Vbias = −230 mV. | ||
A similar but opposite situation is revealed for the enantiomer (S)-2. Domains with a CCW rotation are more stable in this case and are hence formed in 1-heptanol. As (R)-2-octanol is responsible for the stabilization of CCW domains, no chirality inversion is expected to occur, which is confirmed by the data (Table 1). Conversely, (S)-2-octanol stabilizes CW domains. In this chiral solvent approximately 18% of the domains are rotated CW and are thus induced by the solvent (Fig. 3B and C).
For (S,S)-3 with two stereogenic centres at the 5 and 10 positions of the porphyrin ring only CCW domains were observed in (R)-2-octanol. In (S)-2-octanol, 27 domains were probed of which only one domain reveals chirality inversion (+7°). In the case of compound (S,S)-4 that contains two stereogenic centres at the 5 and 15 positions of the macrocycle, irrespective of the nature of the chirality of the solvent, exclusively CCW domains are formed.
Based on the fact that monolayer self-assembly only occurs in protic solvents, a second option would be hydrogen bond formation between the carbonyl group of the porphyrin and the hydroxyl proton of the chiral solvent. MD simulations have indicated that the formation of hydrogen bonds for all chiral porphyrin derivatives (every side chain bears a methyl group and is thus chiral) plays a stabilizing role in the self-assembly process.12 Upon hydrogen bonding the orientation of the area around the amide group could be altered. It seems reasonable to assume that there will be an energetic barrier for the change in orientation. In addition, it is logical that this barrier would increase upon enhancing the number of stereogenic centres. Hence, for porphyrin 1, (R)-2 and (S)-2 it appears that this barrier can be overcome, while porphyrin (S,S)-3 and (S,S)-4 struggle to prevail over this energetic barrier (and only occasionally succeed in doing so). The organization of the porphyrins on the substrate leaves no space for other molecules to adsorb.11 The unit cell parameters remain unchanged upon monolayer formation from a chiral solution, ruling out co-adsorption of the chiral solvent on the graphite surface. Alkyl chains can be resolved clearly in high-resolution STM images, both for monolayers formed in 1-heptanol and the chiral solvents. In all cases, the interdigitated alkyl chains are fully extended and densely packed, with no room for solvent adsorption.
In conclusion, STM experiments have confirmed that chiral solvents can play a major role in the self-assembly process of both achiral and chiral molecules at an interface, and therefore a Sergeants-and-Corporals effect has been observed. Both (S)- and (R)-2-octanol were able to bias the self-assembly of an achiral porphyrin molecule favouring the formation of one of the enantiomorphous structures, probably via molecule–solvent interactions by hydrogen bonds with the amide groups in the surface anchored system. The monolayer chirality of a chiral porphyrin with one stereogenic centre, which forms an enantiomorphous monolayer in 1-heptanol, can even be inverted. When more than one stereogenic centre is present in the porphyrin the molecular chirality dominates over the solvent effect. It is remarkable that this weak and dynamic interaction is able to overcome the effect of a stereogenic centre, and may point the way to the preparation of bistable systems in which organisations of two chiralities are possible and could be interchanged.
This work was supported by the Fund of Scientific Research–Flanders (FWO), KU Leuven (GOA) and the Belgian Federal Science Policy Office (IAP-7/05). I.D. and E.G. are grateful to the Agency for Innovation by Science and Technology in Flanders (IWT). The research leading to these results has received funding from the European Community's Seventh Framework Programme under grant agreement no. NMP4-SL-2008-214340, project RESOLVE, the Generalitat de Catalunya (2009 SGR 158) and the MINECO (CTQ2010-16339).
Notes and references
- (a) L. Pérez-Garcia and D. B. Amabilino, Chem. Soc. Rev., 2007, 36, 941 RSC; (b) Y. Mastai, Chem. Soc. Rev., 2009, 38, 772 RSC; (c) H. Xu, W. J. Saletra, P. Iavicoli, B. Van Averbeke, A. P. H. J. Schenning, D. Beljonne, R. Lazzaroni, D. B. Amabilino and S. De Feyter, Angew. Chem., Int. Ed., 2012, 51, 11981 CrossRef CAS.
- (a) R. M. Hazen and D. S. Sholl, Nature, 2003, 2, 367 CrossRef CAS; (b) T. Mallat, E. Orglmeister and A. Baiker, Chem. Rev., 2007, 107, 4863 CrossRef CAS.
- (a) K. H. Ernst, Top. Curr. Chem., 2006, 265, 209 CrossRef CAS; (b) K. H. Ernst, Curr. Opin. Colloid Interface Sci., 2008, 13, 54 CrossRef CAS; (c) J. A. A. W. Elemans, I. De Cat, H. Xu and S. De Feyter, Chem. Soc. Rev., 2009, 38, 722 RSC; (d) R. Raval, Chem. Soc. Rev, 2009, 38, 707 RSC.
- K. E. Plass, A. L. Grzesiak and A. Matzger, Acc. Chem. Res., 2007, 40, 287 CrossRef CAS.
- (a) P. Samori, X. Yin, N. Tchebotareva, Z. Wang, T. Pakula, F. Jäckel, M. D. Watson, A. Venturini, K. Müllen and J. Rabe, J. Am. Chem. Soc., 2004, 126, 3567 CrossRef CAS; (b) M. M. S. Abdel-Mottaleb, E. Gomar-Nadal, M. Surin, H. Uji-I, W. Mamdouh, J. Veciana, V. Lemaur, C. Rovari, J. Cornil, R. Lazzaroni, D. B. Amabilino, S. De Feyter and F. C. De Schryver, J. Mater. Chem., 2005, 15, 4601 RSC.
- S. M. Barlow and R. Raval, Surf. Sci. Rep., 2003, 50, 201 CrossRef CAS.
- M. M. Green, M. P. Reidy, R. J. Johnson, G. Darling, D. J. Oleary and G. Willson, J. Am. Chem. Soc., 1989, 111, 6452 CrossRef.
- (a) M. Parschau, S. Romer and K. H. Ernst, J. Am. Chem. Soc., 2004, 126, 15398 CrossRef CAS; (b) S. Haq, N. Liu, V. Humblot, A. P. J. Jansen and R. Raval, Nat. Chem., 2009, 1, 409 CrossRef CAS; (c) K. Tahara, H. Yamaga, E. Ghijsens, K. Inukai, J. Adisoejoso, M. O. Blunt, S. De Feyter and Y. Tobe, Nat. Chem., 2011, 3, 714 CrossRef CAS; (d) F. Masini, N. Kalashnyk, M. M. Knudsen, J. R. Cramer, E. Laegsgaard, F. Besenbacher, K. V. Gothelf and T. R. Linderoth, J. Am. Chem. Soc., 2011, 133, 13910 CrossRef CAS; (e) I. De Cat, Z. X. Guo, S. J. George, E. W. Meijer, A. P. H. J. Schenning and S. De Feyter, J. Am. Chem. Soc., 2012, 134, 3171 CrossRef CAS; (f) T. Chen, W. Yang and L. Wan, Nat. Commun., 2013, 4, 1389 CrossRef.
- M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2008, 130, 606 CrossRef CAS.
- (a) N. Katsonis, H. Xu, R. M. Haak, T. Kudernac, Z. Tomovic, S. George, M. Van der Auweraer, A. P. H. J. Schenning, E. W. Meijer, B. L. Feringa and S. De Feyter, Angew. Chem., Int. Ed., 2008, 47, 4997 CrossRef CAS; (b) I. Destoop, E. Ghijsens, K. Katayama, K. Tahara, K. S. Mali, Y. Tobe and S. De Feyter, J. Am. Chem. Soc., 2012, 134, 19568 CrossRef CAS.
- P. Iavicoli, H. Xu, L. N. Feldborg, M. Linares, M. Paradinas, S. Stafström, C. Ocal, B. Nieto-Ortega, J. Casado, J. T. López Navarrete, R. Lazzaroni, S. De Feyter and D. B. Amabilino, J. Am. Chem. Soc., 2010, 132, 9350 CrossRef CAS.
- M. Linares, P. Iavicoli, K. Psychogyiopoulou, D. Beljonne, S. De Feyter, D. B. Amabilino and R. Lazzaroni, Langmuir, 2008, 24, 9566 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, high-resolution STM images of porphyin (S,S)-3 and (S,S)-4. See DOI: 10.1039/c3cc42584c |
| ‡ These authors contributed equally. |
| § For compound (R)-2 one domain with a CCW rotation and for compound (S)-2 two domains with a CW rotation are observed in 1-heptanol. |
| This journal is © The Royal Society of Chemistry 2013 |
