 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Angular dependence of the exchange interaction in fluoride-bridged GdIII–CrIII complexes†
Saurabh Kumar
Singh‡
a,
Kasper S.
Pedersen‡
b,
Marc
Sigrist
bc,
Christian Aa.
Thuesen
b,
Magnus
Schau-Magnussen
b,
Hannu
Mutka
c,
Stergios
Piligkos
b,
Høgni
Weihe
b,
Gopalan
Rajaraman
*a and
Jesper
Bendix
*b
aDepartment of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076, India. E-mail: rajaraman@chem.iitb.ac.in; Fax: +91-22-2576-7152; Tel: +91-22-2576-7183
bDepartment of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen, Denmark. E-mail: bendix@kiku.dk; Fax: +45 35320214; Tel: +45 3520111
cInstitut Laue-Langevin, 38042 Grenoble Cedex 9, France
First published on 1st May 2013
Abstract
The observed angular variation of the magnetic exchange coupling parameter in a series of fluoride-bridged chromium(III)–gadolinium(III) complexes is explained by DFT calculations.
The relatively new research field of magnetic refrigeration, by means of adiabatic demagnetisation, in paramagnetic, molecular, polynuclear, complexes1 and MOFs2 has reinvigorated the study of magnetically isotropic, polynuclear d- and f-metal ion compounds.3 As the magnetocaloric effect (MCE) delicately depends on the nature of the low-lying energy levels of such systems, it is necessary to develop methodologies for the prediction of the sign and magnitude of the magnetic exchange interactions between their constituent metal ions.4 GdIII has a well-isolated, orbitally non-degenerate 8S7/2 ground term and can be considered magnetically isotropic.5 Similarly, CrIII has only weak magnetic anisotropy.6 Thus, a combination of these two metal-ions for the development of new molecular refrigerants seems promising. We have recently reported a penta-nuclear, trigonal bipyramidal {Gd3Cr2} complex displaying good refrigeration properties.7 We report here magneto-structural correlation studies on a simple dinuclear CrIII–GdIII model complex and three, previously described, polynuclear GdIII–CrIII complexes (Fig. 1).8 Characteristically, only very small differences in the CrIII–F and GdIII–F bond lengths are observed in the series 1–4 suggesting that the differences in exchange interactions are essentially governed by the GdIII–F–CrIII angle. The synthetic approach to the series is based on the kinetic robustness of CrIII which prevents the precipitation of highly insoluble GdF3.9 The intrinsic preference of the fluoride ion for linear bridging facilitates the prediction of the cluster topology, giving rods for trans-difluorido, squares for cis-difluorido and trigonal bipyramids for fac-trifluorido chromium(III) precursors.
 | ||
| Fig. 1 Molecular structures of 1–4 (see also Scheme S1, ESI†). Gd, purple; Cr, dark green; F, light green; O, red; N, blue. The auxiliary ligand sphere is shown as a wireframe and hydrogens are omitted for clarity. | ||
The χT products of 1–4 are shown in Fig. 2 of which the magnetic properties of 2–4 have already been reported.7,8b The high-temperature χT values are all in agreement with the values expected for the uncorrelated ions (SGd = 7/2, SCr = 3/2, g = 2.0 for both CrIII and GdIII). On cooling, all χT products decrease indicating the presence of intra-cluster antiferromagnetic interactions. All thermodynamic magnetometry data were fitted by use of the Levenberg–Marquardt algorithm10 and by numerical diagonalisation of the isotropic spin-Hamiltonian (1):
 | (1) |
 | (2) |
 | ||
| Fig. 2 (left) χT (χ = M/H) data for 1–4 acquired with Hdc = 1000 Oe (circles) and best fits as described in the main text (solid lines). (right) INS spectra of 1 obtained with an incident neutron wavelength of λi = 6.5 Å at T = 1.5 K and 15 K. Solid black lines are simulations. | ||
The obtained J parameters for 2–4, for which the INS spectra were not acquired, are shown together with that for 1 in Fig. 3 as a function of the Gd–F–Cr angle. All the extracted couplings are antiferromagnetic and their strength seems to correlate with the Gd–F–Cr angle. Magnetic interactions in {3d–Gd} dinuclear complexes are in general ferromagnetic with some notable exceptions, such as a cyanide-bridged {CrGd} complex.12,13 A {GdCr4} hydroxide bridged complex is also reported to be antiferromagnetic in nature.14 This fuelled our curiosity as to whether CrIII in particular promotes antiferromagnetic interactions. Indeed, for 1–4, the exchange interaction is antiferromagnetic. Moreover, there is a structural correlation to this antiferromagnetic interaction as shown in Fig. 3, which presents J as a function of the Gd–F–Cr angle. To shed light on this issue, as well as on the angular dependence of J, we performed DFT (B3LYP/TZV) calculations on 1. All the calculations were performed with the Gaussian 09 program suite (cf. ESI† for computational details). The computed J value of 0.80 cm−1 for 1 is in excellent agreement with the values extracted from experiments (see Table S2 for DFT computed energies, ESI†). The reproduction of both the sign and the magnitude of J provides confidence in the computed J values.
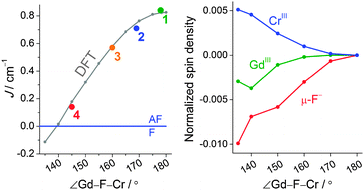 | ||
| Fig. 3 (left) Angular dependence on the J parameters for 1–4 (coloured circles) and DFT results (grey circles). (right) Normalized spin density plot for an angular distortion of 1. | ||
Understanding the mechanism by which the coupling is operational in this {Cr–Gd} pair is important, as it is expected to provide clues about the nature of the exchange interaction and its angular dependence. The following generic mechanistic points emerge from our earlier studies.15a–f In a general {3d–4f} pair, the overlap between the 4f orbitals and the 3d orbitals only partly determines the nature of the magnetic exchange interaction. Orthogonality between the 3d and 4f orbitals results in ferromagnetic, JF, contributions, whereas, non-vanishing overlap of the same orbitals results in antiferromagnetic, JAF, contributions to the magnetic exchange. Additionally, charge transfer excitations from a 3d orbital to an empty 5d orbital of the lanthanide contribute to JF. Since the 4f orbitals are contracted, contributions to JAF are generally weak and therefore most of the {3d–4f} pairs exhibit ferromagnetic behaviour. In complex 1, the unpaired electrons on CrIII are located in the t2g {dxy, dxz, dyz} set of orbitals. Since these orbitals exhibit π character, an efficient σ-type charge transfer from 3d to 5d, observed for {CuII(t2g6eg3)–Gd} and {Ni(t2g6eg2)–Gd} complexes, is absent in 1 and thus, the JF contribution is significantly reduced.15a–c To estimate the JAF contributions, the overlap integrals (Sab) between the magnetic orbitals of CrIII and GdIII have been computed and significant Sab values have been detected indicating a relatively strong 3d–4f overlap (Fig. S6, ESI†). Previously, we have shown that the number of bridges plays a vital role in the sign of magnetic coupling. In particular, when going from two alkoxo-bridges to one bridge in {3d–Gd} complexes, a strong antiferromagnetic interaction is predicted, essentially due to a larger direct 3d–4f orbital overlap and thus a large JAF contribution.15b This correlates well with the single fluoride bridge and the antiferromagnetic interaction observed in this series. Additional insight into the mechanism of the exchange interaction is provided by DFT-calculated spin densities. Spin densities of 3.09 on CrIII and 7.03 GdIII indicate predominant spin polarisation (Fig. 4, left; Table S3, ESI†). All the coordinated nitrogen and oxygen atoms have negative spin densities, however the fluoride ions, both the bridging and the terminal, have positive spin densities in the high spin state (cf. Table S4, ESI†). The bridging fluoride has negative spin density values in the broken symmetry state and this essentially indicates that delocalization from CrIII predominates over the GdIII. Comparing the magnitude and the sign of the spin densities at the fluoride bridge, it is apparent that a mixture of spin delocalization and polarization is operational on these atoms and this diminishes the charge transfer component and thus reduces the JF part. This is also supported by our NBO analysis where an extremely weak CrIII 3dz2–GdIII 5dz2 donor–acceptor interaction has been detected (Fig. 4, right). To gain insight into the angle dependency, the Cr–F–Gd angle was gradually varied from 135° to 180° in steps of 10°. The computed variation of J with the bridging angle is shown in Fig. 3 along with the experimental points.
 | ||
| Fig. 4 (left) Spin density plot for the parent Cr–Gd complex. (right) Representation of charge transfer donor(CrIII 3dz2)–acceptor(GdIII 5dz2) NBO plot from second order perturbation theory analysis. | ||
As the angle increases the J becomes more antiferromagnetic, but shows a plateau at larger angles. On the other hand at an acute angle (at ca. 138°), a switch from antiferro- to ferromagnetic interaction is predicted. Mapping the experimental J's and structural parameters on the computed graph yields an excellent match. The plateau at higher angles and less antiferromagnetic J at lower angles was nicely reproduced. To analyse the factors behind the observed trend, we have computed the overlap integrals, which reveal that there is a significant decrease in the Cr–Gd orbital overlap as the angle decreases leading to a reduction in the JAF contribution. (cf. Table S5, ESI†) This along with a moderate increase in the charge transfer (cf. 5d occupation in Table S6, ESI†) leads to a predicted ferromagnetic coupling at lower Cr–F–Gd angles. Our analysis reveals that at lower angles, the metal ion spin densities increase and, concomitantly, for μ-F− the spin densities decrease proportionally indicating an increasing ferromagnetic contribution as evidenced from the computed J values (see Fig. 3, right; Table S4, ESI†).
In conclusion, the angular dependence of the exchange in a small family of fluoride-bridged {GdIIIxCrIIIy} complexes has been analysed. The observed variation and magnitude of J is reproduced and rationalised by DFT calculations in terms of geometric variation of 3d–4f overlap and charge transfer. This insight paves the way for preparative control of magnetic exchange interactions in systems with small magnetic anisotropy and thereby for the development of new molecular, magnetic coolers.
K.S.P. and S.P. thank the Danish Ministry of Science, Innovation and Higher Education for an EliteForsk travel scholarship and a Sapere Aude Fellowship (10-081659), respectively. GR thanks DST (SR/S1/IC-41/2010; SR/NM/NS-1119/2011) for financial support and SKS thanks IITB for SRF fellowship. GR and SKS acknowledge the HPC facility at IITB.
Notes and references
- (a) M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672–4676 RSC; (b) J. W. Sharples and D. Collison, Polyhedron, 2013, 54, 91–103 CrossRef CAS.
- (a) G. Lorusso, M. A. Palacios, G. S. Nichol, E. K. Brechin, O. Roubeau and M. Evangelisti, Chem. Commun., 2012, 48, 7592–7594 RSC; (b) R. Sibille, T. Mazet, B. Malaman and M. François, Chem.–Eur. J., 2012, 18, 12970 CrossRef CAS.
- (a) R. E. P. Winpenny, Chem. Soc. Rev., 1998, 27, 447–452 RSC; (b) C. Benelli and D. Gatteschi, Chem. Rev., 2002, 102, 2369–2388 CrossRef CAS; (c) M. Andruh, J.-P. Costes, C. Diaz and S. Gao, Inorg. Chem., 2009, 48, 3342–3359 CrossRef CAS.
- E. Cremades, S. Gómez-Coca, D. Aravena, S. Alvarez and E. Ruiz, J. Am. Chem. Soc., 2012, 134, 10532–10542 CrossRef CAS.
- O. Kahn, Molecular Magnetism, VCH, New York, 1993 Search PubMed.
- H. I. Karunadasa, K. D. Arquero, L. A. Berben and J. R. Long, Inorg. Chem., 2010, 49, 4738–4740 CrossRef CAS.
- T. Birk, K. S. Pedersen, C. Aa. Thuesen, T. Weyhermüller, M. Schau-Magnussen, S. Piligkos, H. Weihe, S. Mossin, M. Evangelisti and J. Bendix, Inorg. Chem., 2012, 51, 5435–5443 CrossRef CAS.
- (a) J. Dreiser, K. S. Pedersen, C. Piamonteze, S. Rusponi, Z. Salman, Md. E. Ali, M. Schau-Magnussen, C. Aa. Thuesen, S. Piligkos, H. Weihe, H. Mutka, O. Waldmann, P. Oppeneer, J. Bendix, F. Nolting and H. Brune, Chem. Sci., 2012, 3, 1024–1032 RSC; (b) C. Aa. Thuesen, K. S. Pedersen, M. Schau-Magnussen, M. Evangelisti, J. Vibenholt, S. Piligkos, H. Weihe and J. Bendix, Dalton Trans., 2012, 41, 11284–11292 RSC.
- (a) A. McRobbie, A. R. Sarwar, S. Yeninas, H. Nowell, M. L. Baker, D. Allan, M. Luban, C. A. Muryn, R. G. Pritchard, R. Prozorov, G. Timco, F. Tuna, G. F. S. Whitehead and R. E. P. Winpenny, Chem. Commun., 2011, 47, 6251–6253 RSC; (b) J. Dreiser, K. S. Pedersen, T. Birk, M. Schau-Magnussen, C. Piamonteze, S. Rusponi, T. Weyhermüller, H. Brune, F. Nolting and J. Bendix, J. Phys. Chem. A, 2012, 116, 7842–7847 CrossRef CAS.
- W. H. Press, S. A. Teukolsky, W. T. Vetterling and B. P. Flannery, Numerical Recipes in C: The Art of Scientific Computing, Cambridge University Press, Cambridge, MA, 2nd edn, 1992 Search PubMed.
- P. L. W. Tregenna-Piggott, D. Sheptyakov, L. Keller, S. I. Klokishner, S. M. Ostrovsky, A. V. Palii, O. S. Reu, J. Bendix, T. Brock-Nannestad, K. Pedersen, H. Weihe and H. Mutka, Inorg. Chem., 2009, 48, 128–137 CrossRef CAS.
- (a) N. Georgopoulou, R. Adam, C. P. Raptopoulou, V. Psycharis, R. Ballesteros, B. Abarca and A. K. Boudalis, Dalton Trans., 2010, 39, 5020–5027 RSC; (b) M. Ferbinteanu, F. Cimpoesu, M. A. Gîrţu, C. Enachescu and S. Tanase, Inorg. Chem., 2012, 51, 40–50 CrossRef CAS.
- H.-Z. Kou, S. Gao, C.-H. Li, D.-Z. Liao, B.-C. Zhou, R.-J. Wang and Y. Li, Inorg. Chem., 2002, 41, 4756–4762 CrossRef CAS.
- D. J. Hodgson, K. Michelsen and E. J. Pedersen, J. Chem. Soc., Chem. Commun., 1988, 1558–1559 RSC.
- (a) S. K. Singh, N. K. Tibrewal and G. Rajaraman, Dalton Trans., 2011, 40, 10897–10906 RSC; (b) S. K. Singh and G. Rajaraman, Dalton Trans., 2013, 42, 3623–3630 RSC; (c) G. Rajaraman, F. Totti, A. Bencini, A. Caneschi, R. Sessoli and D. Gatteschi, Dalton Trans., 2009, 3153–3161 RSC; (d) J. Paulovic, F. Cimpoesu, M. Ferbinteanu and K. Hirao, J. Am. Chem. Soc., 2004, 126, 3321–3331 CrossRef CAS; (e) T. Rajeshkumar and G. Rajaraman, Chem. Commun., 2012, 48, 7856–7858 RSC; (f) S. K. Singh, T. Rajeshkumar, V. Chandrasekhar and G. Rajaraman, Polyhedron, 2013 DOI:10.1016/j.poly.2013.02.037.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, crystallographic details, spectroscopic results, reduced magnetization data, computational details. CCDC 921006. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3cc42552e |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2013 |
