Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
NHC–Cu(I) catalysed asymmetric conjugate silyl transfer to unsaturated lactones: application in kinetic resolution†
Vittorio
Pace‡
,
James P.
Rae‡
,
Hassan Y.
Harb
and
David J.
Procter
*
School of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: david.j.procter@manchester.ac.uk
First published on 15th April 2013
Abstract
The scope of the asymmetric silyl transfer to unsaturated lactones utilising a C2-symmetric NHC–Cu(I) catalyst has been established and kinetic resolutions mediated by silyl transfer have been used to prepare enantiomerically enriched anti-4,5-disubstituted 5-membered lactones. The method has been exploited in an expedient synthesis of (+)-blastmycinone.
The asymmetric conjugate addition of a silicon nucleophile to α,β-unsaturated carbonyl compounds is a valuable transformation in organic synthesis1 as the resulting β-silyl carbonyl compounds are robust synthetic equivalents of β-hydroxy carbonyl compounds courtesy of the stereospecific Fleming–Tamao oxidation.2 The β-hydroxy carbonyl moiety is synonymous with the aldol reaction and is a well-known motif: the ability to efficiently generate this valuable motif in stereoselective fashion remains an important goal in modern synthetic chemistry. Fleming's conjugate addition of silyl cuprates to electron-deficient alkenes remained for a long time the method of choice for establishing the β-silyl carbonyl motif.2e,3 In the 1980s Hayashi and Ito pioneered the development of asymmetric silylation using palladium catalysis, however narrow substrate scope has limited adoption of the method.4 In 2006, Oestreich disclosed an asymmetric conjugate silylation protocol using a Si–B reagent (PhMe2SiBpin)1a,5 in the presence of an enantiomerically pure rhodium catalyst.6 In 2010, a complementary protocol from Hoveyda employed N-heterocyclic carbene (NHC) ligands7 in Cu(I)-catalysed asymmetric addition of PhMe2SiBpin to α,β-unsaturated carbonyl systems.8 Hoveyda's strategy exploits the use of readily accessible pre-catalysts with inexpensive Cu(I) salts and thus represents a highly attractive method for asymmetric conjugate silylation.9
Although the Oestreich and Hoveyda protocols allow the asymmetric silylation of a wide range of cyclic and acyclic substrates (e.g. esters, ketones, nitriles), limited studies on silyl transfer to heterocyclic systems, and in particular α,β-unsaturated lactone substrates, have been described.6,8 Furthermore, in general, asymmetric conjugate additions to 5-membered substrates are known to be challenging.10 In this Communication we describe our studies to optimise and establish the scope of the Cu-catalysed asymmetric silyl transfer to unsaturated lactones. We also report the kinetic resolution of 5-substituted butenolides11 mediated by the silyl transfer.
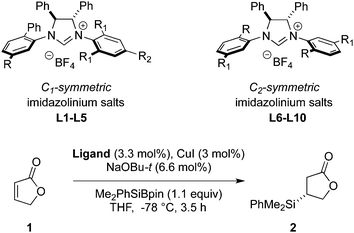
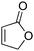
Building on Oestreich's studies involving furanone 1,6a,c we began our investigation by studying silyl transfer to 1 using Hoveyda's protocol.12 Employing L1, a ligand that has previously been used in conjugate silyl transfer to carbocyclic systems (Table 1),8a poor enantiocontrol was observed in the silylation of 1 (entry 1). Subtle modifications of the C1-symmetric imidazolinium salt core did little to improve the outcome (entries 2–5). For furanone 1, enantioselectivities were improved by a switch to C2-symmetric ligands (entries 6–10). In particular, the best results were obtained using C2-symmetric ligands bearing extended aromatic systems (naphthyl and anthryl, entries 8 and 10) on the N-phenyl substituent. However, the presence of additional steric bulk had a detrimental effect on enantioinduction (entry 9). Thus, the use of a C2-symmetric ligand L8 gave the best results in silyl transfer to 1.
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Ligand | Type | R | R1 | R2 | er |
| 1 | L1 | C 1 | Me | Et | H | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 2 | L2 | C 1 | H | Et | H | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
| 3 | L3 | C 1 | i-Pr | Et | H | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| 4 | L4 | C 1 | H | Me | Me | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 5 | L5 | C 1 | Me | Me | Me | 66.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.5 33.5 |
| 6 | L6 | C 2 | H | Ph | — | 80.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.5 19.5 |
| 7 | L7 | C 2 | Me | Ph | — | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 8 | L8 | C 2 | 2-Naphthyl | H | — | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 9 | L9 | C 2 | 2-Naphthyl | i-Pr | — | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
| 10 | L10 | C 2 | 2-Anthryl | H | — | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
With ligand choice completed, we turned our attention to further optimising the reaction conditions (Table 2). Although different Cu(I) sources13 did not have a significant effect on enantioinduction, copper salt selection was important in maximizing conversions due to their moisture sensitivity.
|
|
||||
|---|---|---|---|---|
| Entry | Cu salt | Additive | Conv. (%) | er |
| 1 | CuCl | — | 71 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 2 | CuBr | — | 80 | 90.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5 9.5 |
| 3 | CuBr·SMe2 | — | 68 | 89.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 10.5 |
| 4 | CuOTf | — | 91 | 89.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 10.5 |
| 5 | CuI | — | >98 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 6 | CuI | 4 Å MS | >98 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
In this sense, the use of CuI gave the best results (entry 5) and the addition of molecular sieves improved reproducibility. Importantly, we found that ‘glove-box free’ conditions could be used thus greatly simplifying operational procedures. With optimised conditions in hand, we applied the protocol to the asymmetric conjugate silylation of 5, 6 and 7-membered α,β-unsaturated lactones (Table 3). The desired β-silyl adducts were obtained in up to 96.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
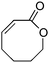
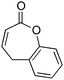
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 er in good yield (entries 1–3). Interestingly, the 8-membered lactone did not react, presumably due to conformational effects (entry 4). When a 7-membered lactone was fused with an aromatic ring, there was a deleterious effect upon enantiocontrol although the yield remained high (entry 5). Moderate selectivity was also observed in the silylation of a 2-substituted lactone (entry 6).
3.5 er in good yield (entries 1–3). Interestingly, the 8-membered lactone did not react, presumably due to conformational effects (entry 4). When a 7-membered lactone was fused with an aromatic ring, there was a deleterious effect upon enantiocontrol although the yield remained high (entry 5). Moderate selectivity was also observed in the silylation of a 2-substituted lactone (entry 6).
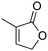
To further explore the scope of the protocol, the kinetic resolution of a series of 5-substituted butenolides was carried out using Cu(I)–NHC catalysed asymmetric silyl transfer.14 Pleasingly, treatment of 5-substituted butenolides with 60–70 mol% of PhMe2SiBpin and the C2-symmetric catalyst derived from L8 and CuI afforded silylated products after kinetic resolution in good yields (up to a maximum of 50%), good enantiomeric ratios and as single anti-diastereoisomers (Table 4).15
|
|
|||||
|---|---|---|---|---|---|
| Entry | Substrate | Conversiona (%) | Yieldb (%) | er | s |
| a Determined by 1H NMR. b Yield of isolated product. c Selectivity factor determined according to ref. 16. d 10 mol% catalyst loading, 0.7 equiv. (PhMe2SiBpin). | |||||
| 1 |
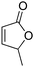
|
50 | 46 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
13 |
| 2 |
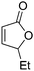
|
52 | 50 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
25 |
| 3d |
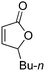
|
43 | 43 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
19 |
| 4d |
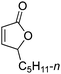
|
48 | 43 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
12 |
| 5 |
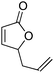
|
47 | 46 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
10 |
| 6d |
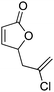
|
49 | 48 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
18 |
| 7d |
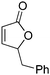
|
43 | 42 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
11 |
| 8 |
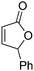
|
45 | 41 | 88.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.5 11.5 |
15 |
The rate of addition to 5-substituted butenolides was slower than silyl transfer to unsubstituted lactones, presumably due to increased steric hindrance, therefore higher catalyst and silylborane loading was required.17 Primary alkyl, allyl, benzyl and phenyl substituents at the 5-position of butenolides were found to be compatible with the process. To our knowledge, these examples represent the first kinetic resolutions achieved by Cu-catalysed silyl transfer from a Si–B reagent.
To demonstrate the value of Cu(I)–NHC catalysed asymmetric silyl transfer to unsaturated lactones, we report a concise approach to (+)-blastmycinone, a natural product arising from the hydrolysis of the antibiotic (+)-antimycin A3 (Scheme 1).18 Alkylation of silylated lactone 3 (see entry 1, Table 4) provided 4 with three contiguous stereocenters as a single diastereoisomer. Fleming–Tamao oxidation2 then gave lactone 5, which after esterification afforded (+)-blastmycinone.19
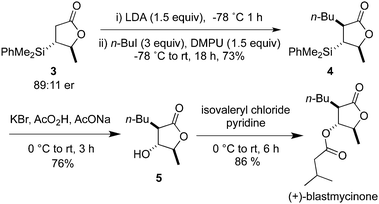 | ||
| Scheme 1 Catalytic asymmetric approach to (+)-blastmycinone. | ||
In summary, we have explored the scope of a convenient procedure for asymmetric silyl transfer to unsaturated lactones. The Cu(I)–NHC catalysed process delivers β-silylated lactones in good yields and enantioselectivities. In contrast to observations with other substrate classes, the use of C2-symmetric imidazolinium salts as NHC precursors was crucial for efficient asymmetric silyl transfer to unsaturated 5-membered lactones. Kinetic resolution using Cu-catalysed silyl transfer from a Si–B reagent has been applied to racemic 5-butenolides and affords products with good enantiocontrol and excellent diastereocontrol. The method has been used in an expedient asymmetric synthesis of (+)-blastmycinone.
We thank The Leverhulme Trust (V.P.) and the EPSRC (J.P.R.) for funding and Robyn Bullough for assistance optimising the conversion of 3–5.
Notes and references
- For selected reviews, see: (a) M. Oestreich, E. Hartmann and M. Mewald, Chem. Rev., 2013, 113, 402 CrossRef CAS; (b) E. Hartmann, D. J. Vyas and M. Oestreich, Chem. Commun., 2011, 47, 7917 RSC; (c) M. Suginome and Y. Ito, Chem. Rev., 2000, 100, 3221 CrossRef CAS; (d) E. Hartmann and M. Oestreich, Chim. Oggi, 2011, 29, 34 CAS.
- (a) I. Fleming, R. Henning and H. Plaut, J. Chem. Soc., Chem. Commun., 1984, 29 RSC; (b) I. Fleming and P. E. J. Sanderson, Tetrahedron Lett., 1987, 28, 4229 CrossRef CAS; (c) K. Tamao, N. Ishida, T. Tanaka and M. Kumada, Organometallics, 1983, 2, 1694 CrossRef CAS; (d) K. Tamao, T. Tanaka, T. Nakajima, R. Sumiya, H. Arai and Y. Ito, Tetrahedron Lett., 1986, 27, 3377 CrossRef CAS; (e) I. Fleming, A. Barbero and D. Walter, Chem. Rev., 1997, 97, 2063 CrossRef CAS; (f) I. Fleming, in Science of Synthesis, ed. I. Fleming, Thieme, Stuttgart, 2002, vol. 4, pp. 927–946 Search PubMed.
- For other approaches to racemic β-silyl carbonyls see: (a) L. Iannazzo and G. A. Molander, Eur. J. Org. Chem., 2012, 4923 CrossRef CAS; (b) B. H. Lipshutz, J. A. Sclafani and T. Takanami, J. Am. Chem. Soc., 1998, 120, 4021 CrossRef CAS; (c) G. Auer, B. Weiner and M. Oestreich, Synthesis, 2006, 2113 CAS; (d) H. Ito, T. Ishizuka, J.-i. Tateiwa, M. Sonoda and A. Hosomi, J. Am. Chem. Soc., 1998, 120, 11196 CrossRef CAS; (e) C. T. Clark, J. F. Lake and K. A. Scheidt, J. Am. Chem. Soc., 2004, 126, 84 CrossRef CAS; (f) M. Oestreich and B. Weiner, Synlett, 2004, 2139 CrossRef CAS.
- (a) T. Hayashi, Y. Matsumoto and Y. Ito, J. Am. Chem. Soc., 1988, 110, 5579 CrossRef CAS; (b) Y. Matsumoto, T. Hayashi and Y. Ito, Tetrahedron, 1994, 50, 335 CrossRef CAS.
- For a review of Si–B chemistry, see: T. Ohmura and M. Suginome, Bull. Chem. Soc. Jpn., 2009, 82, 29 CrossRef CAS.
- (a) C. Walter, G. Auer and M. Oestreich, Angew. Chem., Int. Ed., 2006, 45, 5675 CrossRef CAS; (b) C. Walter and M. Oestreich, Angew. Chem., Int. Ed., 2008, 47, 3818 CrossRef CAS; (c) C. Walter, R. Fröhlich and M. Oestreich, Tetrahedron, 2009, 65, 5513 CrossRef CAS.
- For NHCs in metal catalysis, see: (a) S. Díez-González, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef; (b) S. Díez-González and S. P. Nolan, Aldrichimica Acta, 2008, 41, 43 Search PubMed; (c) F. Glorius, Topics in Organometallic Chemistry, N-Heterocyclic Carbenes in Transition Metal Catalysis, Springer-Verlag, Berlin, Heidelberg, 2006, vol. 21, pp. 1–218 Search PubMed.
- (a) K.-S. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2010, 132, 2898 CrossRef CAS; (b) K.-S. Lee, H. Wu, F. Haeffner and A. H. Hoveyda, Organometallics, 2012, 31, 7823 CrossRef CAS ; For a metal-free catalytic C–Si bond formation, see: J. M. O'Brien and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7712 Search PubMed.
- For alternative asymmetric approaches to β-silyl carbonyl compounds, see: (a) I. Ibrahem, S. Santoro, F. Himo and A. Córdova, Adv. Synth. Catal., 2011, 353, 245 CrossRef CAS; (b) A. Welle, J. Petrignet, B. Tinanat, J. Wouters and O. Riant, Chem.–Eur. J., 2010, 16, 10980 CrossRef CAS; (c) B. H. Lipshutz, N. Tanaka, B. R. Taft and C.-T. Lee, Org. Lett., 2006, 8, 1963 CrossRef CAS; (d) R. Shintani, K. Okamoto and T. Hayashi, Org. Lett., 2005, 7, 4757 CrossRef CAS; (e) M. A. Kacprzynski, S. A. Kazane, T. L. May and A. H. Hoveyda, Org. Lett., 2007, 9, 3187 CrossRef CAS.
- (a) S. Kehrli, D. Martin, D. Rix, M. Mauduit and A. Alexakis, Chem.–Eur. J., 2010, 16, 9890 CrossRef CAS; (b) D. Martin, S. Kehrli, M. d'Augustin, H. Clavier, M. Mauduit and A. Alexakis, J. Am. Chem. Soc., 2006, 128, 8416 CrossRef CAS; (c) L. Liang, T. T.-L. Au-Yeung and A. S. C. Chan, Org. Lett., 2002, 4, 3799 CrossRef CAS.
- (a) For a reductive dynamic kinetic resolution of similar substrates, see: M. P. Rainka, J. E. Milne and S. L. Buchwald, Angew. Chem., Int. Ed., 2005, 44, 6177 CrossRef CAS; (b) For kinetic resolutions of similar substrates using organolithiums, see: S. H. Lim and P. Beak, Org. Lett., 2002, 4, 2657 CrossRef CAS.
- For a preliminary result, see: H. Y. Harb, K. D. Collins, J. V. G. Altur, S. Bowker, L. Campbell and D. J. Procter, Org. Lett., 2010, 12, 5446 CrossRef CAS.
- The use of CuCN was ineffective.
- (a) For the kinetic resolution of racemic 1-alkyl-2-methylenecyclopropanes using PhMe2SiBpin and Pd-catalysis, see: T. Ohmura, H. Taniguchi and M. Suginome, Org. Lett., 2009, 11, 2880 CrossRef CAS; (b) For the two-directional desymmetrization of α,β-unsaturated esters using PhMe2SiBpin and Rh-catalysis, see: E. Hartmann and M. Oestreich, Org. Lett., 2012, 14, 2406 CrossRef CAS.
- I. Fleming, N. L. Reddy, K. Takaki and A. C. Ware, J. Chem. Soc., Chem. Commun., 1987, 1472 RSC.
- (a) E. Vedejs and M. Jure, Angew. Chem., Int. Ed., 2005, 44, 3974 CrossRef CAS; (b) H. B. Kagan and J. C. Fiaud, Top. Stereochem., 1988, 18, 249 CrossRef CAS.
- Iso-propyl 5-substituted butenolide resulted in low conversion (ca. 10%) but good enantiocontrol (er 87
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13). Attempted addition to the analogous tert-butyl substituted lactone resulted in no conversion.
13). Attempted addition to the analogous tert-butyl substituted lactone resulted in no conversion. - M.-J. Chen, C.-Y. Lo, C.-C. Chin and R.-S. Liu, J. Org. Chem., 2000, 65, 6362 CrossRef CAS.
- R. S. Ferrarini, A. A. Dos Santos and J. V. Comasseto, Tetrahedron, 2012, 68, 10601 CrossRef CAS and references cited therein.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc42160k |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |