 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dual-purpose PEG scaffolds for the preparation of soft and biofunctional hydrogels: the convergence between CuAAC and thiol–ene reactions†
Kim
Öberg
a,
Yvonne
Hed
a,
Isabella
Joelsson Rahmn
a,
Jonathan
Kelly
b,
Peter
Löwenhielm
bc and
Michael
Malkoch
*a
aKTH Royal Institute of Technology, Department of Fibre and Polymer Technology, Teknikringen 56-68, SE-100 44 Stockholm, Sweden. E-mail: malkoch@kth.se; Fax: +46 8 790 8283; Tel: +46 8 790 8768
bMölnlycke Health Care AB, Box 130 80, SE 40252 Gothenburg, Sweden
cSP Technical Research Institute of Sweden, Box 857, SE 501 15 Borås, Sweden
First published on 17th April 2013
Abstract
Orthogonally functionalized PEGs displaying alkenes and azides have been prepared and their dual-purpose scaffolding potential was exploited via click chemistry for controlled insertion of biorelevant moieties as well as facile fabrication of soft, non-toxic and degradable hydrogels.
Poly(ethylene glycol) (PEG) based hydrogels are promising 3D networks for a range of biomedical applications including tissue engineering,1 drug delivery,2 and as artificial extra cellular matrices (aECMs) for studying matrix-influenced cell behaviour.3 Their biocompatibility along with their hydrophilic and elastic nature makes them attractive materials for producing high water content reservoirs that mimic natural ECMs. Traditional PEG hydrogels are prepared via non-controlled radical initiated chain growth polymerization of acrylic end-functional prepolymers. However, to offer superior properties in terms of uniform network structures and improved mechanical performance, step-growth approaches capitalizing on robust chemical reactions such as copper-catalyzed azide–alkyne cycloaddition (CuAAC) click reaction and UV initiated thiol–ene coupling (TEC) reaction are nowadays preferred.4,5 Furthermore, the inherent inertness of PEGs commonly requires addition of biorelevant moieties to enhance their cell-interaction efficacy in which bioorthogonal click chemistries have emerged as a powerful tool.6 Strategies allowing unprecedented control over both presentation of biochemical cues and network physiochemical properties are highly desired. One elegant strategy to introduce a high degree of functionality within hydrogels while maintaining precise control over the 3D architecture has been to use dendritic–linear–dendritic (DLD) hybrids. In fact, such scaffolds have also been evaluated for improved gene transfection,7 ionically crosslinked self-healing hydrogels8 and adhesives for corneal wound repair.9 In this report, bifunctional PEGs are prepared via a dendritic hybridization approach and their dual-purpose function evaluated for the construction of soft and bioactive hydrogels.
PEGs, 6 kDa and 10 kDa, were selected as model components for the introduction of dual functionalities, Scheme 1. For the bifunctionalization, anhydride activated AB2C-type monomer 1 was used to efficiently produce the desired scaffolds.10,11 Esterification reactions of the two PEGs with 4 equimolar excess of 1, followed by mild acidic deprotection afforded P6k-[G1]-(OH)4-(N3)25 and P10k-[G1]-(OH)4-(N3)26. Both compounds were isolated by simple purification protocols and their architectural integrity was supported by NMR and MALDI-ToF MS as can be seen in Fig. S2, (ESI†). To exploit these scaffolds for the fabrication of functional hydrogels, the four terminal hydroxyl groups were reacted with anhydride 2 and monitored by MALDI. The resulting P6k-[G1]-(ene)4-(N3)27 (Fig. 1) and P10k-[G1]-(ene)4-(N3)28 decorated with four peripheral enes and two azides were isolated in excellent yields. The versatility of this concept was further explored to generate functional PEG scaffolds with a higher and controlled number of dual-purpose sites. In this case, P6k-[G1]-(OH)48 with four terminal hydroxyl groups was treated in a similar fashion resulting in a dendritic–linear–dendritic (DLD) block-copolymer P6k-[G2]-(ene)8-(N3)412. A pure DLD polymer was obtained in three reaction steps with a total yield of 63% and displayed two-fold increased orthogonal functionalities i.e. eight ene and four azide groups, Fig. S3, (ESI†). While these scaffolds were targeted for the fabrication of functional hydrogels, it is important to point out that the dual functionalities can accurately be modified for other multipurpose studies.
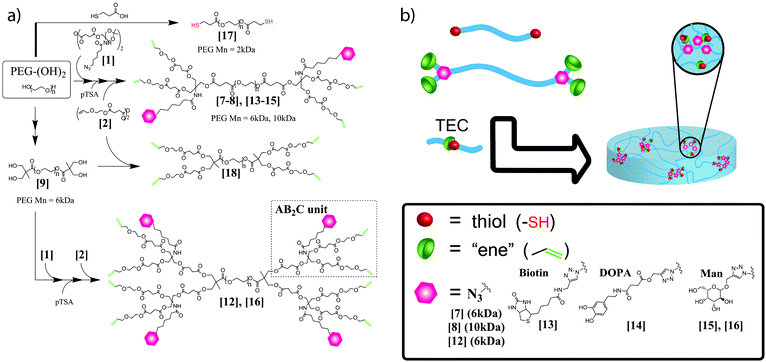 | ||
| Scheme 1 (a) Introducing dual crosslinkable and bioactive functionalities to PEGs and (b) hydrogel fabrication. | ||
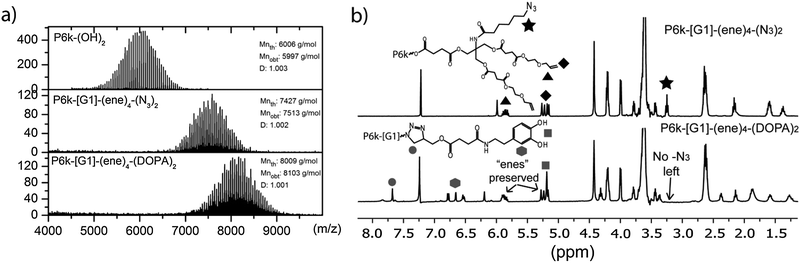 | ||
| Fig. 1 (a) MALDI-ToF and (b) 1H NMR used to follow CuAAC coupling to P6k-G1-ene4-(N3)2 with alkyne functional DOPA. | ||
The orthogonal nature of these scaffolds was assessed towards chemoselective modifications of the azide functionalities with biorelevant moieties (BMs) via the robust CuAAC click reaction.12 The azides were reacted with alkyne functional biotin, L-dopamine (DOPA) and D-mannose (Man) at room temperature under THF–water conditions in the presence of sodium ascorbate and copper sulfate. For complete conversion of the azide groups a [PEG-N3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BM-alkyne]
[BM-alkyne]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cu]
[Cu]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ascorbate] ratio of 1
[ascorbate] ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 was found to be optimal resulting in scaffolds 13, 14 and 15. As shown in Fig. 1, the attachments of dopamines proceed with high efficacy producing the targeted compound 14 in the range of the expected theoretical molecular weights and with narrow dispersity. The completion of these reactions were furthermore confirmed using 1H NMR by the disappearance of the methylene group (–CH2N3) at 3.24 ppm, Fig. 1b.
0.4 was found to be optimal resulting in scaffolds 13, 14 and 15. As shown in Fig. 1, the attachments of dopamines proceed with high efficacy producing the targeted compound 14 in the range of the expected theoretical molecular weights and with narrow dispersity. The completion of these reactions were furthermore confirmed using 1H NMR by the disappearance of the methylene group (–CH2N3) at 3.24 ppm, Fig. 1b.
One crucial parameter when utilizing CuAAC for producing biomaterials is the removal of catalytic copper to avoid any copper induced toxicity.13 The concentration of copper in drinking water should be below 1.3 ppm14 and for in vitro culturing of hepatocyte cells the TD50 (toxic-dose-50) is in the level of 48 ppm (750 μM).15 The initial copper concentration in the crude P6k-[G1]-(ene)2-(Man)215 was calculated to be 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 ppm, which is over 700 times above the threshold TD50 levels found in the mentioned in vitro studies. Interestingly, a simple purification protocol was identified which included purging through a neutral Al2O3 column followed by a dialysis treatment in 0.5% (w/w) EDTA (pH 7.0). Graphite furnace atomic adsorption spectroscopy (GF-AAS) of the purified compounds 13–16 revealed a substantial [Cu] reduction to acceptable concentrations of 3.5 to 11.5 ppm.
760 ppm, which is over 700 times above the threshold TD50 levels found in the mentioned in vitro studies. Interestingly, a simple purification protocol was identified which included purging through a neutral Al2O3 column followed by a dialysis treatment in 0.5% (w/w) EDTA (pH 7.0). Graphite furnace atomic adsorption spectroscopy (GF-AAS) of the purified compounds 13–16 revealed a substantial [Cu] reduction to acceptable concentrations of 3.5 to 11.5 ppm.
The motivation for the construction of functional hydrogels was to mimic body tissues of various Young's modulus, ranging from soft brain tissue of 0.1–1 kPa to crosslinked osteoid collagen of 25–40 kPa.16 UV initiated TEC chemistry has recently been shown to be a robust method for the construction of hydrogels with gel fractions of 95% and above.5 As a result, 2 kDa PEG with dithiol functionalities P2K-(SH)217 was synthesized and used as a flexible crosslinker for the fabrication of hydrogels. Four different networks were prepared via UV initiated TEC chemistry in ethanol (E) or water (W) with a balanced molar ratio between the [ene] and [SH] of dual-functional azide–ene PEGs 7, 8, 12 and the crosslinker 17. All hydrogels, H6k-[G1]-(N3)2-E, H10k-[G1]-(N3)-E and H6k-[G2]-(N3)4-E and H6k-[G1]-(N3)2-W, were efficiently produced within 10 minutes upon a cytocompatible UV exposure of 365 nm (9 mW cm−2) and from 30 wt% of dry materials including photoinitiator I2959.17 As shown in Fig. 2, both the length of PEG and the dendron generation (G1 and G2) have a dramatic effect on the modulus and swelling properties. The Young's moduli (E) were found to be similar to several body tissues ranging from 3.5 kPa for the H10k-[G1]-(N3)2 gel and up to 27 kPa for H6k-[G2]-(N3)4-E. These values were further corroborated by swelling profiles ranging from 800 wt% for the densely crosslinked H6k-[G2]-(N3)4-E to 2000 wt% swelling for the softer H10k-[G1]-(N3)2, Fig. S4, (ESI†).
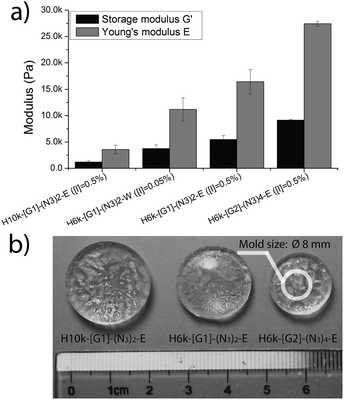 | ||
| Fig. 2 (a) Obtained moduli and (b) water swelling properties. | ||
Satisfied with the facile methodology for the construction of soft networks with azide functionalities, hydrogels were prepared in 24-well cell culturing plates. Five hydrogels; H6k-[G2]-(Man)4 and three H6k-[G1]-(X)2 (X = N3, DOPA and Man), with sub-ppm copper concentrations, Table S1, (ESI†), as well as neutral H6k-[G1]-N (from P6k-[G1], 18) were constructed using the PEG2k-(SH)2 crosslinker, at 30 wt% dry content in aqueous media and with [I2959] = 0.05 wt%.
After a thorough washing procedure, their potential use as in vitro cell scaffolds was evaluated. Normal dermal human fibroblast cells (6k cells per well) were seeded topologically and the cell viability was measured over 7 days by a lactate dehydrogenase (LDH) assay, Fig. 3. In all cases, the LDH levels were in the range of the control containing only cell culturing medium. Intriguingly, the exposure of fibroblast cells to all G1 hydrogels resulted in full structural degradation within 3 days. This phenomenon was not observed for hydrogels exposed to cell culturing media without cells under equivalent conditions. The H6k-[G2]-(Man)4 network was found to be more stable and required 7 days to fully degrade. The detected non-toxicity demonstrates the feasibility of these hydrogels as temporary aECMs during the recovery of damaged soft tissue. A comprehensive and in-depth study of affinity of cells vs. hydrogels decorated with larger dendrons and higher loading of an array of multivalent BMs, including ECM adhesive peptides, is on-going and will be described in future publications.
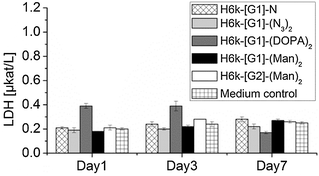 | ||
| Fig. 3 Viability measured by LDH assay for five PEG–dendron hydrogels with medium control. The detection limit was 0.17 μkat L−1. | ||
In summary, a robust methodology for the construction of bifunctionalized PEGs has been proposed. Their dual-purpose scaffolding ability via CuAAC click chemistry and UV initiated TEC chemistry resulted in the fabrication of bioactive hydrogels with moduli in the range of several body tissues. The apparent degradability of the networks coupled with the low cytotoxicity towards dermal human fibroblast cells may render them suitable as impermanent and aECMs.
We acknowledge Vinnova-Sambio and the Swedish Research Council VR (2011-5358 and 2010-453) for financial support. Dr Sofia Almqvist is acknowledged for the cell viability study.
Notes and references
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- A. S. Sawhney, C. P. Pathak and J. A. Hubbell, Macromolecules, 1993, 26, 581–587 CrossRef CAS.
- A. M. Kloxin, A. M. Kasko, C. N. Salinas and K. S. Anseth, Science, 2009, 324, 59–63 CrossRef CAS.
- M. Malkoch, R. Vestberg, N. Gupta, L. Mespouille, P. Dubois, A. F. Mason, J. L. Hedrick, Q. Liao, C. W. Frank, K. Kingsbury and C. J. Hawker, Chem. Commun., 2006, 2774–2776 RSC.
- T. Yang, M. Malkoch and A. Hult, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 363–371 CrossRef CAS.
- M. A. Azagarsamy and K. S. Anseth, ACS Macro Lett., 2013, 2, 5–9 CrossRef CAS.
- L. Albertazzi, F. M. Mickler, G. M. Pavan, F. Salomone, G. Bardi, M. Panniello, E. Amir, T. Kang, K. L. Killops, C. Braeuchle, R. J. Amir and C. J. Hawker, Biomacromolecules, 2012, 13, 4089–4097 CAS.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339–343 CrossRef CAS.
- M. W. Grinstaff, Biomaterials, 2007, 28, 5205–5214 CrossRef CAS.
- M. I. Montañez, Y. Hed, S. Utsel, J. Ropponen, E. Malmström, L. Wågberg, A. Hult and M. Malkoch, Biomacromolecules, 2011, 12, 2114–2125 CrossRef.
- P. Antoni, Y. Hed, A. Nordberg, D. Nyström, H. von Holst, A. Hult and M. Malkoch, Angew. Chem., Int. Ed., 2009, 48, 2126–2130 CrossRef CAS.
- P. Antoni, M. J. Robb, L. Campos, M. Montanez, A. Hult, E. Malmstrom, M. Malkoch and C. J. Hawker, Macromolecules, 2010, 43, 6625–6631 CrossRef CAS.
- L. M. Gaetke and C. K. Chow, Toxicology, 2003, 189, 147–163 CrossRef CAS.
- D. J. Fitzgerald, Am. J. Clin. Nutr., 1998, 67, 1098S–1102S CAS.
- M. L. Schilsky, R. R. Blank, M. J. Czaja, M. A. Zern, I. H. Scheinberg, R. J. Stockert and I. Sternlieb, J. Clin. Invest., 1989, 84, 1562–1568 CrossRef CAS.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS.
- S. J. Bryant, C. R. Nuttelman and K. S. Anseth, J. Biomater. Sci., Polym. Ed., 2000, 11, 439–457 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the experimental procedures and synthesis. See DOI: 10.1039/c3cc42084a |
| This journal is © The Royal Society of Chemistry 2013 |
