 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Five-fold symmetric penta-substituted corannulene with gelation properties and a liquid-crystalline phase†
Martin
Mattarella‡
a,
Johannes M.
Haberl‡
b,
Janne
Ruokolainen
c,
Ehud M.
Landau
a,
Raffaele
Mezzenga
b and
Jay S.
Siegel§
*a
aInstitute of Organic Chemistry, University of Zurich, Winterthurerstrasse 190, 8057, Zurich, Switzerland. E-mail: jss@oci.uzh.ch; Tel: +41 446354281
bFood & Soft Materials, Department of Health Science and Technology, ETH Zurich, Schmelzbergstrasse 9, 8092, Zurich, Switzerland
cDepartment of Applied Physics, Aalto University School of Science, 00076, Aalto, Finland
First published on 17th June 2013
Abstract
Five-fold symmetric substituted corannulene derivatives that display liquid-crystalline behavior and organogelation properties were prepared by coupling of N-azidoethyl long-chain fatty acidamides to sym-pentabutynyl corannulenes via dipolar cycloaddition chemistry.
Corannulene (1) is a bowl-shaped aromatic system that displays physical properties differing from those of planar π-conjugated analogs.1,2 Its conical symmetry dictates different electron densities on its concave and convex π-faces, the magnitude of which leads to a dipole moment of roughly 5 D;3 therefore, a material comprising derivatives of 1 ordered in a columnar manner might orient in an electric field and show ferroelectric properties.4 So far, columnar order of corannulenes has been observed in the solid state for few corannulene derivatives5 and in the liquid-crystalline mesophase for deca-substituted corannulenes.6 The recent kilogram scale synthesis of corannulene7 and the development of a facile procedure for the preparation of several C5-symmetric penta-substituted corannulenes8 opened the road to synthesize a family of lipid–corannulene conjugates and to screen their physical and chemical properties.
Three sym-pentakislipido-corannulene9 derivatives (2, 3 and 4) are the focus of this study. Chlorination of 1 leads to the sym-pentachlorocorannulene (5),10 which represents the key synthetic intermediate en route to C5-symmetric substituted corannulenes (Scheme 1).11 Iron-catalyzed five-fold coupling of the homopropargyl magnesium agent X with 5 followed by base mediated removal of the terminal TMS groups yields 6. The desired conjugates, 2–4, are then obtained in high yield and purity by CuAAC activated dipolar cycloaddition between 6 and the respective alkyl azide.12
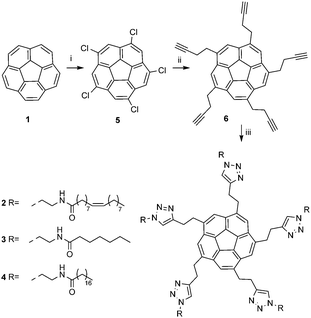 | ||
| Scheme 1 Reaction conditions: (i) ICl, DCM, from −75 °C to r.t., 82 h. (ii) 1. 1-Trimethylsilyl-4-butynyl magnesium bromide, Fe(acac)3, THF/NMP, r.t., 2.5 h. 2. NaOH, MeOH/THF/H2O, r.t., 24 h. (iii) RN3, Cu nanoparticles, DMF, microwave, 60 °C, 2 h. | ||
The phase behavior of 2, 3 and 4 was initially studied by differential scanning calorimetry (DSC) and polarized optical microscopy (POM) (Fig. S1, ESI†). For 2 thermal signatures characteristic of phase transitions were observed at 132 °C and at 99 °C in the DSC cooling scan, and the birefringence texture in the POM images was consistent with the presence of a liquid-crystalline phase. For 3 neither thermal signatures characteristic of phase transition in the DSC scan nor birefringence in the POM images were observed. For 4DSC cooling scans revealed transitions at 80 °C and 30 °C and again, birefringence of the liquid phases indicated the presence of a liquid-crystalline phase, however only beyond the melting at 30 °C.
Small- and wide-angle X-ray scattering experiments (SAXS and WAXS) were performed in order to obtain more information about the supramolecular organization of 2 at room temperature (Fig. 1). In the wide-angle region, an isotropic halo indicates the liquid-like behavior of the component without a specific π-interaction peak (Fig. 1a). In the small angle region, the appearance of a strong reflection followed by a weak second order reflection at q2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) q1 = 2
q1 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggests a lamellar phase (Fig. 1b). The azimuthally integrated X-ray scattered intensity shows the Bragg reflection of lamellae with a layer-to-layer distance of 4.4 nm after sample annealing (Fig. 1c). Transmission electron microscopy (TEM) images of 2 were acquired to visualize the lamellar structure (Fig. 2a) and a very long-range ordered lamellar phase was observed, with a d-spacing of 4.1–4.3 nm measured after Fourier transformation, in good agreement with the X-ray analysis.
1 suggests a lamellar phase (Fig. 1b). The azimuthally integrated X-ray scattered intensity shows the Bragg reflection of lamellae with a layer-to-layer distance of 4.4 nm after sample annealing (Fig. 1c). Transmission electron microscopy (TEM) images of 2 were acquired to visualize the lamellar structure (Fig. 2a) and a very long-range ordered lamellar phase was observed, with a d-spacing of 4.1–4.3 nm measured after Fourier transformation, in good agreement with the X-ray analysis.
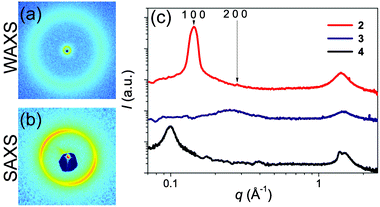 | ||
| Fig. 1 (a) 2D wide and (b) small angle X-ray scattering (WAXS, SAXS) patterns of 2 and (c) the 1D scattering intensity distribution of 2, 3 and 4 after annealing. | ||
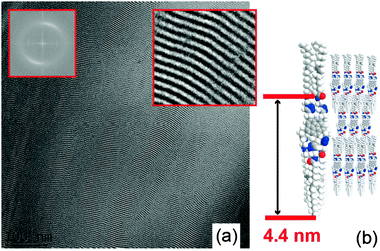 | ||
| Fig. 2 (a) Transmission electron microscopy (TEM) image of compound 2 with a lamellar structure. The insets show the Fourier transformation (FFT) and a zoom-up of a lamellar domain (edge size of 50 × 45 nm). (b) A possible molecular model of the molecular organization in the lamellar structure. | ||
The gelation properties of 2 in organic solvent, IPA–water and ethanol–water mixtures were screened in order to study its propensity for molecular aggregation.13,14 In cyclohexane (1 w/w%) and methylcyclohexane (2 w/w%), 2 forms gels with fully thermoreversible behavior upon cooling after being heated to 60 °C. Analysis by POM and SWAXS of the gel in cyclohexane did not show any birefringent phase or ordered structure.
To get further information about the orientation of corannulene cores with respect to each other UV-vis and emission spectroscopies14 were performed (Fig. S2 and S3, ESI†). Quenching of the fluorescence was found with increasing concentration of 2. However, the absorption spectra do not show any hypsochromic or bathochromic shift, which augurs poorly for the existence of π-stacked structures in the gel as well as H- and J-aggregates.
To study the influence of hydrogen bonding on the formation of the gel, IR spectra were measured as a function of concentration of 2 in cyclohexane.13 Upon increasing concentration of 2 and gelation, neither of the amide bands (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N–H) were shifted (Table S4, ESI†). Thus, no evidence for the formation of hydrogen bonds in the gelation process was found. These findings for 2 suggest a molecular model in which the microphase separation of the corannulene core and the symmetric substituents drive the molecule into a lamellar structure with partially interdigitated alkyl chains (Fig. 2b) and reduced symmetry compared to the molecule itself. In the presence of solvent, the microstructure of 2 is lost in the gel.
O, N–H) were shifted (Table S4, ESI†). Thus, no evidence for the formation of hydrogen bonds in the gelation process was found. These findings for 2 suggest a molecular model in which the microphase separation of the corannulene core and the symmetric substituents drive the molecule into a lamellar structure with partially interdigitated alkyl chains (Fig. 2b) and reduced symmetry compared to the molecule itself. In the presence of solvent, the microstructure of 2 is lost in the gel.
SAXS and WAXS studies on 3, bearing a short saturated tail, reveal only a diffuse peak, indicative of a correlation hole at 2.5 nm and corresponding to chemical heterogeneities on the scale of the molecule.15 Furthermore, 3 does not show any gelation behavior and is solely observed as an isotropic liquid.
The SAXS and WAXS studies on 4, the direct saturated analog of 2, reveal the presence of a partially crystallized phase at room temperature (Fig. 1c). In cyclohexane (1 w/w%) 4 forms gels with fully thermoreversible behavior upon cooling after being heated to 60 °C. Similar to compound 2, FTIR, UV-vis and emission spectra of gels of 4 in cyclohexane suggest the absence of any ordered supramolecular organization or H-bonding network in the gel phase.13
In summary, these results show that the nature of the hydrocarbon chains influences the phase behavior and the gelation properties of C5-symmetric pentakislipido-corannulene derivatives. Specifically, 2, functionalized with oleyl chains, forms gels in cyclohexane and, as a neat substance, exhibits a room temperature liquid-crystalline phase, with lamellar order and a 4.4 nm layer-to-layer spacing. In contrast, 3, with short saturated chains showed no appreciable LC or gelation properties, and 4 appears in a partially crystallized phase and forms gel only in cyclohexane. The promising results for 2 and the versatility of the synthetic strategy provoke one to consider a wide library of molecularly engineered sym-penta-substituted corannulenes suitable for numerous materials applications.
Notes and references
- For review, see: Y.-T. Wu and J. S. Siegel, Chem. Rev., 2006, 106, 4843 Search PubMed.
- T. J. Sieders, K. K. Baldridge, G. H. Grube and J. S. Siegel, J. Am. Chem. Soc., 2001, 123, 517 CrossRef.
- K. K. Baldridge and J. S. Siegel, Theor. Chem. Acc., 1997, 97, 67 CrossRef CAS.
- L. M. Blinov, Liq. Cryst., 1998, 24, 143 CrossRef CAS; J. L. Atwood, L. J. Barbour, M. J. Hardie and C. Raston, Coord. Chem. Rev., 2001, 222, 3 CrossRef CAS; B. Xu and T. M. Swager, J. Am. Chem. Soc., 1993, 115, 1159 CrossRef CAS; D. Mizajima, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, Science, 2012, 336, 209 Search PubMed.
- Y.-T. Wu, T. Hayama, K. K. Baldrige, A. Linden and J. S. Siegel, J. Am. Chem. Soc., 2006, 128, 6870 CrossRef CAS; A. Sygula, H. E. Folsom, R. Sygula, A. H. Abdourazak, Z. Marcinow, F. R. Fronczek and P. W. Rabideau, J. Chem. Soc., Chem. Commun., 1994, 2571 RSC; Y.-T. Wu, D. Bandera, R. Maag, A. Linden, K. K. Baldridge and J. S. Siegel, J. Am. Chem. Soc., 2008, 130, 10729 CrossRef CAS; B. M. Schmidt, S. Seki, B. Topolinski, K. Ohkubo, S. Fukuzumi, H. Sakurai and D. Lentz, Angew. Chem., Int. Ed., 2012, 51, 11385 Search PubMed; I. V. Kuvychko, S. N. Spisak, Y.-S. Chen, A. A. Popov, M. A. Petrukhina, S. H. Strauss and O. V. Boltalina, Angew. Chem., Int. Ed., 2012, 51, 4939 Search PubMed.
- D. Miyajima, K. Tashiro, F. Araoka, H. Takezoe, J. Kim, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2009, 131, 44 CrossRef CAS.
- A. M. Butterfield, B. Gilomen and J. S. Siegel, Org. Process Res. Dev., 2012, 16, 664 Search PubMed.
- M. Mattarella and J. S. Siegel, Org. Biomol. Chem., 2012, 10, 5799 RSC.
- Prefix sym is used here to indicate the C5-symmetric-substituted corannulene corresponding to 1,3,5,7,9-pentasubstitution.
- T. J. Seiders, E. L. Elliot, G. H. Grube and J. S. Siegel, J. Am. Chem. Soc., 1999, 121, 7804 CrossRef CAS.
- A new candidate as a starting point for the synthesis of sym-penta-substituted corannulenes has been recently reported: M. N. Eliseeva and L. T. Scott, J. Am. Chem. Soc., 2012, 134, 15169 Search PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC.
- ESI†.
- M. Wang, G. L. Silva and B. A. Armitage, J. Am. Chem. Soc., 2000, 122, 9977 CrossRef CAS; E. G. McRae, Aust. J. Chem., 1961, 14, 229; E. Jelley, Nature, 1936, 138, 1009 CrossRef CAS; E. Jelley, Nature, 1937, 139, 631 CrossRef CAS.
- L. Leibler, Macromolecules, 1980, 13, 1602 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details, 1H and 13C NMR and gelation tests for compounds 2–4. POM, DSC, UV-vis and emission spectra of 2 and 4. See DOI: 10.1039/c3cc41476k |
| ‡ These authors contributed equally. |
| § Current address: School of Pharmaceutical Science and Technology, Tianjin University, A210/Bldg 24, 92 Weijin Road, Nankai District, Tianjin, 300072 PR China. E-mail: dean_spst@tju.edu.cn. |
| This journal is © The Royal Society of Chemistry 2013 |
