 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Is Y2(B12H12)3 the main intermediate in the decomposition process of Y(BH4)3?†
Yigang
Yan
*a,
Arndt
Remhof
a,
Daniel
Rentsch
b,
Young-Su
Lee
c,
Young
Whan Cho
c and
Andreas
Züttel
a
aEMPA, Swiss Federal Laboratories for Materials Science and Technology, Hydrogen & Energy, 8600 Dübendorf, Switzerland. E-mail: yigang.yan@empa.ch; Fax: +41 58 765 40 22; Tel: +41 58 765 40 82
bEMPA, Swiss Federal Laboratories for Materials Science and Technology, Functional Polymers, 8600 Dübendorf, Switzerland
cHigh Temperature Energy Materials Research Center, Korea Institute of Science and Technology, 136-791 Seoul, Republic of Korea
First published on 17th April 2013
Abstract
Dodecaborates, i.e. the [B12H12]2− containing species, are often observed as main intermediates in the hydrogen sorption cycle of metal borohydrides, hindering rehydrogenation. In the decomposition process of Y(BH4)3, yttrium octahydrotriborate, i.e. Y(B3H8)3, rather than the stable Y2(B12H12)3, is formed as the main intermediate.
Metal borohydrides M(BH4)n (n is the valence of metal M), with high gravimetric (18.4 wt% for LiBH4) and volumetric (102 kg m−3 for LiBH4) hydrogen densities, have been widely investigated for hydrogen storage in recent years.1,2 The stability of M(BH4)n was reported to depend on the charge transfer from M to [BH4], and thereby a correlation between the electronegativity (χp) of metal M and the stability of the corresponding M(BH4)n was established.3 Applying this empirical rule to Y (χp = 1.2), a decomposition temperature of 500 K, well below those of alkaline and alkaline earth metal borohydrides, is expected for Y(BH4)3. Combined with a hydrogen content of 9.1 wt%, it makes Y(BH4)3 an attractive candidate for solid hydrogen storage.
Experimentally, Y(BH4)3 was observed to release hydrogen below 200 °C.4–6 The whole hydrogen release process occurs in multistep reactions with the formation of amorphous intermediates. Within the hydrogen sorption cycle, the reaction pathway and the intermediates involved determine the reversibility. For example, dodecaborates (i.e. the [B12H12]2− species), which are unwanted by-products and reduce the rehydrogenation performance, are often observed as intermediates in the decomposition process of alkaline and alkaline earth metal borohydrides.7–14 F. C. Gennari reported the observation of the [B12H12]2− species using Fourier transform infrared spectroscopy during the decomposition of Y(BH4)3.15 However, no further characterization was reported to support the formation of Y2(B12H12)3. Here, we demonstrate that Y(BH4)3 decomposes via the formation of Y(B3H8)3, and only traces of Y2(B12H12)3 are detected using solution state 11B nuclear magnetic resonance (NMR). Besides, Y2(B12H12)3 was synthesized by ball milling Y(BH4)3 with B2H6 and its stability was examined.
The dehydrogenation of Y(BH4)3 (prepared according to ref. 6) was carried out by temperature programmed desorption (TPD) under a constant flow of 1, 5 and 10 bar H2, respectively. The samples dehydrogenated at 240 to 350 °C were dissolved in D2O and examined using 11B NMR. Fig. 1a shows the solution state 11B(1H) NMR spectra of Y(BH4)3 decomposed under 1 bar H2. A main resonance at −30.8 ppm together with some additional minor resonances is observed in the samples decomposed at 240, 255 and 280 °C, while no dissolved intermediates are observed in the sample decomposed at 350 °C. The chemical shift of −30.8 ppm agrees well with that of octahydrotriborate (i.e. the [B3H8]− species) reported in the literature.16–18 In the 2D 1H–11B HMQC NMR spectrum of Y(BH4)3 after heating to 255 °C (Fig. 1b), a single correlation signal of [B3H8]− centered at 0.2 ppm (1H) and −30.8 ppm (11B) is observed. This single resonance is explained by the fact that all three boron atoms are equivalent and that each one couples with all eight of the hydrogen atoms.16 In addition, the minor resonance in Fig. 1a at 19.6 ppm assigned to boric acid is attributed to the hydrolysis product of the remaining Y(BH4)3 in D2O. The minor resonances at −15.2, −16.1 and −17.3 ppm suggest the existence of [B12H12]2− or its derivatives [B12H12−n(OH)n]2− (n = 1 to 4),19 and those at −8.4, −22.7 and −36.2 ppm are possibly related to the formation of [B10H14]2−.20
 | ||
| Fig. 1 (a) Solution state (D2O) 11B(1H) NMR spectra (128.38 MHz) of Y(BH4)3 decomposed at 240 to 350 °C; (b) two dimensional 1H–11B HMQC NMR spectrum of Y(BH4)3 decomposed at 255 °C. | ||
The applied H2 external pressure is known to influence the decomposition pathway of metal borohydrides.13,21 Therefore, the experiment was repeated at 5 and 10 bar external H2 pressures. The decomposition products were dissolved in D2O and the corresponding 11B(1H) NMR spectra are shown in Fig. S1 (ESI†). Y(B3H8)3 is also identified as the main phase in Y(BH4)3 decomposed at 300 °C under both 5 and 10 bar, and no dissolved intermediate species are observed in the samples decomposed at 400 °C. As a consequence, the above observations indicate that regardless of H2 external pressures, Y(BH4)3 decomposes via the formation of Y(B3H8)3 as the main intermediate according to eqn (1).
| Y(BH4)3 → 1/3Y(B3H8)3 + 2/3YH3 + H2 | (1) |
| 2Y(BH4)3 + 15B2H6 → Y2(B12H12)3 + 39H2 | (2) |
![11B NMR spectra (128.38 MHz) recorded in DMSO-d6 of the as-prepared Y2(B12H12)3: (a) 1H decoupled and (b) 1H coupled. Coupling constants of 124, 124 and 81 Hz were determined for [B12H12]2−, [B10H10]2− and [BH4]−, respectively.](/image/article/2013/CC/c3cc41184b/c3cc41184b-f2.gif) | ||
| Fig. 2 11B NMR spectra (128.38 MHz) recorded in DMSO-d6 of the as-prepared Y2(B12H12)3: (a) 1H decoupled and (b) 1H coupled. Coupling constants of 124, 124 and 81 Hz were determined for [B12H12]2−, [B10H10]2− and [BH4]−, respectively. | ||
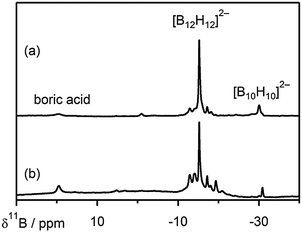 | ||
| Fig. 3 11B(1H) NMR spectra (128.38 MHz) recorded in D2O of (a) the as-prepared Y2(B12H12)3 and (b) Y2(B12H12)3 after heating to 450 °C for 5 h. | ||
| Compound | DMSO-d6 | D2O | ||
|---|---|---|---|---|
| δ 11B/ppm | Mol% | δ 11B/ppm | Mol% | |
| Y2(B12H12)3 | −15.3 | 60 ± 6 | −15.2 | 42 ± 4 |
| Y2(B10H10)3 | −28.5, −0.4 | 8 ± 1 | −30.0, −0.8 | 7 ± 1 |
| Y(BH4)3 | −35.2 | 32 ± 3 | — | — |
| Boric acid | — | — | 19.6 | 51 ± 5 |
To examine the stability of Y2(B12H12)3, the as-synthesized Y2(B12H12)3 was heated up to 450 °C under a constant flow of 1 bar H2. Only limited hydrogen (1.4 wt%) is released in this process, as shown in Fig. S2 (ESI†), compared to the theoretical hydrogen capacity of 6.0 wt% of Y2(B12H12)3. After heating to 450 °C for 5 h, the signal of the [B12H12]2− species still belongs to the main resonance in the solution (D2O) state 11B(1H) NMR spectrum (Fig. 3b), indicating its relatively high stability. The increase in the resonances at −12.8, −14.0, −17.1, −18.0, −19.3, −20.3 and −24.7 ppm assigned to the [B12H12−n(OH)n]2− (n = 1–4) derivatives suggests that the dehydrogenation of Y2(B12H12)3 might proceed through the formation of Y2(B12H12−n)3 adducts.
The formation of higher boranes, such as the [B12H12]2− species, by the reaction of [BH4]− with B2H6 has been reported for LiBH4 and NaBH4.22–24 We demonstrated that this method is also applicable to Y(BH4)3 in the presence of B2H6 yielding Y2(B12H12)3, a species which is stable up to at least 450 °C and readily soluble in water and DMSO-d6. For the widely discussed borohydrides such as LiBH4, NaBH4, Mg(BH4)2 and Ca(BH4)2, the [B12H12]2− species have been identified as the main intermediates in the decomposition process.7–14 In contrast to these borohydrides, Y(BH4)3 shows a different decomposition route. The absence of the dissolved B–H species in Y(BH4)3 decomposed at 350 °C (Fig. 1, top curve) implies that the stable [B12H12]2− species does not play a major role as an intermediate in the decomposition process. Instead, yttrium octahydrotriborate Y(B3H8)3 was formed as the main intermediate.
The [B3H8]− species has been reported as an intermediate favorable for the rehydrogenation of [BH4]− under moderate conditions in the case of Mg(BH4)2.18 Although the reversible hydrogen release amount of Y(BH4)3via the formation of Y(B3H8)3 is not enough to meet the on-board storage target. Considering only traces of the stable [B12H12]2− species formed in the decomposition process (under an external pressure of 1 bar H2), it can be anticipated that Y(BH4)3 can be further developed as a reversible hydrogen storage material under moderate conditions.
Financial support by a grant from Switzerland through the Swiss Contribution to the enlarged European Union and by the Korean Research Council is gratefully acknowledged.
Notes and references
- J. Graetz, Chem. Soc. Rev., 2009, 38, 73 RSC.
- H.-W. Li, Y. Yan, S. Orimo, A. Züttel and C. M. Jensen, Energies, 2011, 4, 185 CrossRef CAS.
- Y. Nakamori, K. Miwa, A. Ninomiya, H. W. Li, N. Ohba, S. Towata, A. Züttel and S. Orimo, Phys. Rev. B, 2006, 74, 45126 CrossRef.
- Y. Yan, H.-W. Li, T. Sato, N. Umeda, K. Miwa, S. Towata and S. Orimo, Int. J. Hydrogen Energy, 2009, 34, 5732 CrossRef CAS.
- D. B. Ravnsbæk, Y. Filinchuk, R. Černý, M. B. Ley, D. Haase, H. J. Jakobsen, J. Skibsted and T. R. Jensen, Inorg. Chem., 2010, 49, 3801 CrossRef.
- A. Remhof, A. Borgschulte, O. Friedrichs, Ph. Mauron, Y. Yan and A. Züttel, Scr. Mater., 2012, 66, 280 CrossRef CAS.
- S. Orimo, Y. Nakamori, N. Ohba, K. Miwa, M. Aoki, S. Towata and A. Züttel, Appl. Phys. Lett., 2006, 89, 021920 CrossRef.
- S. J. Hwang, R. C. Bowman, J. W. Reiter, J. Rijssenbeek, G. L. Soloveichik, J.-C. Zhao, H. Kabbour and C. C. Ahn, J. Phys. Chem. C, 2008, 112, 3164 CAS.
- V. Ozolins, E. H. Majzoub and C. Wolverton, J. Am. Chem. Soc., 2009, 131, 230 CrossRef CAS.
- H.-W. Li, K. Miwa, N. Ohba, T. Fujita, T. Sato, Y. Yan, S. Towata, M. W. Chen and S. Orimo, Nanotechnology, 2009, 20, 204013 CrossRef.
- Y. Kim, S.-J. Hwang, J. Shim, Y.-S. Lee, H. N. Han and Y. W. Cho, J. Phys. Chem. C, 2012, 116, 4330 CAS.
- R. J. Newhouse, V. Stavila, S.-J. Hwang, L. E. Klebanoff and J. Z. Zhang, J. Phys. Chem. C, 2010, 114, 5224 CAS.
- Y. Yan, A. Remhof, S.-J. Hwang, H.-W. Li, Ph. Mauron, S. Orimo and A. Züttel, Phys. Chem. Chem. Phys., 2012, 14, 6514–6519 RSC.
- P. Ngene, R. van den Berg, M. H. W. Verkuijlen, K. P. de Jong and P. E. de Jongh, Energy Environ. Sci., 2011, 4, 4108–4115 CAS.
- F. C. Gennari, Int. J. Hydrogen Energy, 2012, 37, 18895 CrossRef CAS.
- B. M. Graybill, J. K. Ruff and M. F. Hawthorne, J. Am. Chem. Soc., 1961, 83, 2669 CrossRef CAS.
- Z. Huang, G. King, X. Chen, J. Hoy, T. Yisgedu, H. K. Lingam, S. G. Shore, P. M. Woodward and J. C. Zhao, Inorg. Chem., 2010, 49, 8185 CrossRef CAS.
- M. Chong, T. Autrey, S. Orimo, S. Jalisatgi and C. M. Jensen, Chem. Commun., 2011, 47, 1330 RSC.
- T. Peymann, C. B. Knobler and M. F. Hawthorne, Inorg. Chem., 2000, 39, 1163 CrossRef CAS.
- S. Heřmānek, Chem. Rev., 1992, 92, 325 CrossRef.
- Y. Kim, S. J. Hwang, J. H. Shim, Y. S. Lee, H. N. Han and Y. W. Cho, J. Phys. Chem. C, 2012, 116, 4330 CAS.
- H. C. Miller, N. E. Miller and E. L. Muetterties, J. Am. Chem. Soc., 1963, 85, 3885 CrossRef CAS.
- H. C. Miller, N. E. Miller and E. L. Muetterties, Inorg. Chem., 1964, 3, 1456 CrossRef CAS.
- O. Friedrichs, A. Remhof, S.-J. Hwang and A. Züttel, Mater. Chem., 2010, 22, 3265 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of sample preparation and 11B and 1H NMR measurements, solution state 11B(1H) NMR spectra of Y(BH4)3 decomposed under 5 and 10 bar H2 and hydrogen desorption performance of the as-synthesized Y2(B12H12)3. See DOI: 10.1039/c3cc41184b |
| This journal is © The Royal Society of Chemistry 2013 |
