 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bis[zinc(II)dipicolylamino]-functionalised peptides as high affinity receptors for pyrophosphate ions in water†
Karen K. Y.
Yuen
and
Katrina A.
Jolliffe
*
School of Chemistry, The University of Sydney, 2006, NSW, Australia. E-mail: kate.jolliffe@sydney.edu.au; Fax: +61 2 9351 3329; Tel: +61 2 9351 2297
First published on 8th April 2013
Abstract
A library of bis[zinc(II)dipicolylamine]-functionalised linear peptides was prepared using an efficient solid phase peptide synthesis approach and their use as chemosensors for anions in water was investigated using indicator displacement assays. High affinity and selectivity for pyrophosphate (PPi) over adenosine triphosphate (ATP) and adenosine diphosphate (ADP) were observed and additional aromatic side chains provided enhanced discrimination between PPi and ATP.
Anions play fundamental roles in numerous important biological and chemical processes. In particular, phosphate oxoanions such as pyrophosphate (PPi) and adenosine triphosphate (ATP) are involved in a number of bioenergetic and metabolic processes. Therefore there is intense current interest in developing receptors that can efficiently and selectively detect phosphate oxoanions under physiological conditions.1,2
Bis[Zn(II)dipicolylamine] [Zn(II)Dpa] receptors have been found to exhibit good selectivity for phosphates over other anions in aqueous solution.2 In recent studies we have observed that cyclic peptide derived bis[Zn(II)Dpa] receptors can be tuned to show selectivity for PPi over ATP using indicator displacement assays through altering of the cyclic peptide structure.3,4 Notably, both the structure of ‘non-binding’ amino acid side chains decorating the scaffolds and the size of the cyclic peptide have a significant impact on receptor selectivity and affinity.4 In order to further probe the interesting effect of ‘non-binding’ side chains on receptor selectivity we desired rapid access to libraries of peptide scaffolds with varying structures. However, the synthesis and purification of such cyclic peptides is experimentally complex and time-consuming. We envisaged that receptors with simpler linear peptides scaffolds, which should be readily accessible by solid phase synthesis, would provide efficient access to a library of receptors for screening the effects of different side chains on receptor selectivity.
To enable rapid access to a library of receptors, we developed a synthetic route that would enable the entire synthesis, prior to addition of the zinc(II) ions, to be performed on the solid phase. We initially designed receptors 1–7·Zn22 (Fig. 1) containing two or three amino acids and attempted their synthesis using Fmoc solid phase peptide synthesis (Fmoc-SPPS) (Scheme 1). Orthogonal allyloxycarbonyl (Alloc) protecting groups were employed for protection of the side chain amino groups. Following synthesis of the desired linear peptide sequences using standard Fmoc-SPPS protocols on Rink amide resin and acetyl capping of the N-terminal amino acid, the Alloc groups were removed upon treatment with Pd(PPh3)4 (1.05 equiv.) in the presence of acetic acid and morpholine.5 Subsequent reductive aminations with picolylaldehyde6 to install the dipicolylamino groups were only partially successful and in some cases the reaction could not be forced to completion despite subjecting the solid phase bound peptides to repetitive reaction attempts. After analysis of the partially alkylated products following cleavage from the resin, we established that reductive amination of the amino acid directly attached to the resin was not proceeding to completion, presumably as a result of difficulties of reagent access to this hindered amino group. Therefore, for the synthesis of receptors 8–10·Zn222, a C-terminal glycine residue was incorporated as a spacer between the resin and the residue to be reacted. In these cases, the reductive aminations readily proceeded to completion after two reaction cycles, confirming that steric hindrance by the resin was the cause of the previous incomplete conversions. Subsequent cleavage of the peptides from the resin was followed by HPLC purification and addition of two equivalents of Zn(NO3)2 provided receptors 1–10·Zn22.
 | ||
| Fig. 1 Structures of receptors 1–10·Zn22 and indicator 11. | ||
 | ||
| Scheme 1 General method for synthesis of linear peptides on Rink Amide AM resin via Fmoc-SPPS strategy. | ||
The anion binding capabilities of receptors 1–10·Zn22 were investigated using indicator displacement assays (IDAs) with the colourimetric indicator pyrocatechol violet (11), which has previously been employed in IDAs with bis[Zn(II)Dpa] complexes.7 This indicator provides the ability to rapidly screen anion binding to receptor libraries through naked eye visualisation, which can then be followed by measurement of binding affinities by UV-Vis spectroscopy for positive hits. IDAs were performed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
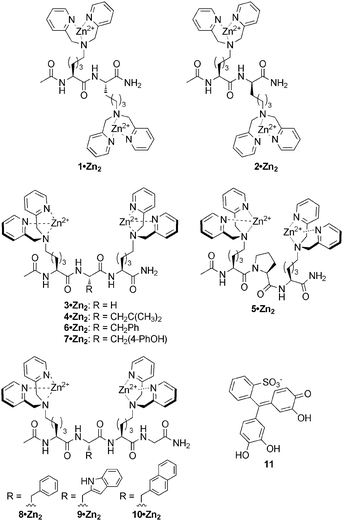
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–indicator chemosensing ensembles prepared by mixing equimolar amounts (20 μM) of receptors 1–10·Zn22 and indicator 11 in buffered saline solution (pH 7.4, 5 mM HEPES, 145 mM NaCl). Upon addition of five equivalents of a range of anions as their sodium salts (PPi, ATP, ADP, AMP, cAMP, pThr, pSer, citrate, SO42− and HPO42−), it was found that in most cases only PPi, ATP and ADP resulted in a colour change indicative of indicator displacement (Fig. 2). UV-Vis spectroscopic analysis of the resulting solutions indicated that in some cases a small change in absorbance was also observed upon addition of citrate, although the change was not large enough to be visible to the naked eye.
1 receptor–indicator chemosensing ensembles prepared by mixing equimolar amounts (20 μM) of receptors 1–10·Zn22 and indicator 11 in buffered saline solution (pH 7.4, 5 mM HEPES, 145 mM NaCl). Upon addition of five equivalents of a range of anions as their sodium salts (PPi, ATP, ADP, AMP, cAMP, pThr, pSer, citrate, SO42− and HPO42−), it was found that in most cases only PPi, ATP and ADP resulted in a colour change indicative of indicator displacement (Fig. 2). UV-Vis spectroscopic analysis of the resulting solutions indicated that in some cases a small change in absorbance was also observed upon addition of citrate, although the change was not large enough to be visible to the naked eye.
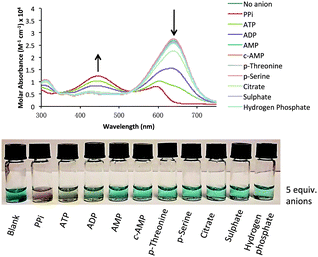 | ||
Fig. 2 Qualitative screening of 9·Zn222·11 after addition of 5 equiv. of anions to a 20 μM receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) indicator solution. UV-vis spectra (top) and visual screening (bottom). indicator solution. UV-vis spectra (top) and visual screening (bottom). | ||
Association constants for the indicator–receptor complexes were determined by UV-Vis spectroscopic titration experiments and non-linear least squares fitting of the titration data to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using Hypspec®.8 (Table 1 and Fig. 3). The 1
1 binding model using Hypspec®.8 (Table 1 and Fig. 3). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was confirmed by Job plot analysis (Fig. 3, bottom). Subsequently, the 1
1 binding stoichiometry was confirmed by Job plot analysis (Fig. 3, bottom). Subsequently, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 indicator
1 indicator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) receptor mixtures were titrated with aliquots of the sodium salts of PPi, ATP and ADP (Fig. 4). Curve fitting of the absorbance data (250–750 nm) based on the previously described equilibria for competition assays9 using Hypspec® provided apparent association constants for the receptor
receptor mixtures were titrated with aliquots of the sodium salts of PPi, ATP and ADP (Fig. 4). Curve fitting of the absorbance data (250–750 nm) based on the previously described equilibria for competition assays9 using Hypspec® provided apparent association constants for the receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anion complexes. In all cases the receptors exhibited very strong binding to PPi (log Ka ranging from 7.8 to 9) in these mimicked physiological conditions and some selectivity for PPi over ATP and ADP was also observed. Of this library of receptors, dipeptide receptor 1·Zn22 was the least effective at discriminating between polyphosphate species. In contrast, dipeptide receptor 2·Zn222, which contains alternating L- and D-amino acids exhibited high affinity and excellent selectivity for PPi over ATP and ADP, indicating the importance of receptor stereochemistry and suggesting that, despite the inherent flexibility in the Lys derived Zn(II)Dpa side chains, positioning these on the same face of the scaffold increases selectivity.
anion complexes. In all cases the receptors exhibited very strong binding to PPi (log Ka ranging from 7.8 to 9) in these mimicked physiological conditions and some selectivity for PPi over ATP and ADP was also observed. Of this library of receptors, dipeptide receptor 1·Zn22 was the least effective at discriminating between polyphosphate species. In contrast, dipeptide receptor 2·Zn222, which contains alternating L- and D-amino acids exhibited high affinity and excellent selectivity for PPi over ATP and ADP, indicating the importance of receptor stereochemistry and suggesting that, despite the inherent flexibility in the Lys derived Zn(II)Dpa side chains, positioning these on the same face of the scaffold increases selectivity.
| Receptor | ‘Spacer’ amino acid | Apparent association constants (log Ka) | |||
|---|---|---|---|---|---|
| Indicator 11 | PPi | ATP | ADP | ||
| a Titrations performed at 25 °C in aqueous solutions buffered at pH 7.4 with 5 mM HEPES in the presence of 145 mM NaCl. Estimated error in log Ka > 0.2. | |||||
| 1·Zn22 | — | 6.6 | 8.7 | 7.2 | 5.8 |
| 2·Zn222 | — | 6.6 | >9 | 6.6 | 5.3 |
| 3·Zn222 | Gly | 5.4 | 7.8 | 5.4 | 4.2 |
| 4·Zn22 | Leu | 7.4 | >9 | 7.1 | 5.3 |
| 5·Zn22 | Pro | 6.8 | >9 | 6.5 | 4.9 |
| 6·Zn22 | Phe | 8.3 | >9 | 8.2 | 5.5 |
| 7·Zn22 | Tyr | 6.9 | >9 | 6.7 | 5.0 |
| 8·Zn22 | Phe | 8.2 | >9 | 7.8 | 5.3 |
| 9·Zn22 | Trp | 7.0 | >9 | 6.4 | 4.6 |
| 10·Zn22 | Nal | 6.1 | >9 | 5.9 | 4.7 |
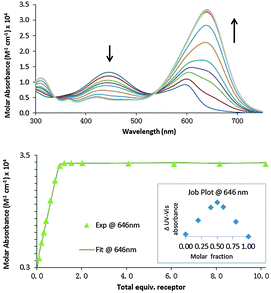 | ||
Fig. 3 Representative spectra showing the change in molar absorbance of indicator 11 (20 μM) upon addition of increasing amounts of 9·Zn22 (top); fitting curve for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between 9·Zn22 and indicator 11 at 646 nm (bottom; Job Plot inset). Measurement conditions: aqueous solution of HEPES buffer (5 mM, pH 7.4, 145 mM NaCl), λabs 250–750 nm, 25 °C. 1 binding between 9·Zn22 and indicator 11 at 646 nm (bottom; Job Plot inset). Measurement conditions: aqueous solution of HEPES buffer (5 mM, pH 7.4, 145 mM NaCl), λabs 250–750 nm, 25 °C. | ||
 | ||
| Fig. 4 Representative spectra showing absorbance changes at 646 nm of 9·Zn222·11 ensemble upon addition of various anions (up to 10 equiv.). Measurement conditions: aqueous solution of HEPES buffer (5 mM, pH 7.4, 145 mM NaCl), λabs 250–750 nm, 25 °C. | ||
We reasoned that the inclusion of a spacer amino acid between two L-configured Zn(II)Dpa residues should provide similar geometrical positioning of the sidechains,10 with an increased space between them which might also provide an increase in binding affinity. As anticipated, most of the tripeptide and tetrapeptide-based receptors (3–10·Zn22) displayed enhanced affinity and selectivity for PPi over ATP and ADP when compared to 1·Zn22 and similar affinities and selectivity to 2·Zn222. The introduction of side chain functionalities on the spacer amino acid was found to have significant effects on both binding affinity and selectivity. Notably, the incorporation of a hydrophobic residue in the peptide sequence (4–10·Zn22) resulted in a substantial increase in affinity for both PPi and ATP, when compared to the affinities obtained for the Gly derivative 3·Zn222. A comparison of the binding data for 6·Zn22 and 8·Zn22 indicates that the addition of a Gly residue at the C-terminus (to facilitate solid phase synthesis) has little effect on binding affinity or selectivity. While 4–10·Zn22 all bound PPi with affinity too high to quantify (log Ka > 9) under the current competitive conditions, analysis of the difference in their affinity for ATP provides some indication of receptor ability to discriminate between ATP and PPi. Incorporation of a Pro residue to provide a more defined peptide backbone conformation did not have a significant impact on binding affinity or selectivity, with similar binding behaviour observed for the Leu (4·Zn22) and Pro (5·Zn22) derivatives. Notably compounds 9·Zn22 and 10·Zn22, which bear large aromatic spacer residues (Trp and Nal, respectively) had reduced affinity for ATP. While the reason for this requires further investigation, these results indicate that the incorporation of hydrophobic and aromatic residues can be used to tune the anion recognition abilities of such scaffolds to provide better discrimination between anions of similar charge.
In conclusion, the design, synthesis, and anion binding capabilities of a library of linear peptide-based receptors (1–10·Zn22), bearing two Zn(II)Dpa binding sites are reported. Receptors 2, 9 and 10·Zn22 show remarkably strong binding to PPi with significant selectivity over ATP and ADP under mimicked physiological conditions. Studies to elucidate the reasons for the enhanced binding selectivities exhibited by receptors with hydrophobic and aromatic side chains are currently in progress.
The Australian Research Council is acknowledged for financial support. KKYY thanks the University of Sydney for the award of a Gritton Postgraduate Scholarship. We thank Dr H. T. Ngo (USyd) for assistance with data analysis using Hyperquad®.
Notes and references
- A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603 CrossRef CAS; P. A. Gale, Chem. Commun., 2011, 47, 82 RSC; S. Kubik, Chem. Soc. Rev., 2010, 39, 3648 RSC; S. K. Kim, D. H. Lee, J.-I. Hong and J. Yoon, Acc. Chem. Res., 2008, 42, 23 CrossRef; J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928 RSC.
- S. J. Butler and K. A. Jolliffe, Org. Biomol. Chem., 2011, 9, 3471 Search PubMed; J. V. Carolan, S. J. Butler and K. A. Jolliffe, J. Org. Chem., 2009, 74, 2992 CrossRef CAS; M. J. McDonough, A. J. Reynolds, G. W. Y. Lee and K. A. Jolliffe, Chem. Commun., 2006, 2971 RSC.
- X. Liu, H. T. Ngo, Z. Ge, S. J. Butler and K. A. Jolliffe, Chem. Sci., 2013, 4, 1680 RSC; S. J. Butler and K. A. Jolliffe, Chem.–Asian. J., 2012, 7, 2621 CrossRef CAS.
- S. A. Kates, S. B. Daniels and F. Albericio, Anal. Biochem., 1993, 212, 303 CrossRef CAS.
- Y. Azuma, H. Imai, T. Yoshimura, T. Kawabata, M. Imanishi and S. Futaki, Org. Biomol. Chem., 2012, 10, 6062 Search PubMed; L. Quinti, R. Weissleder and C.-H. Tung, Nano Lett., 2006, 6, 488 CrossRef CAS.
- M. S. Han and D. H. Kim, Angew. Chem., Int. Ed., 2002, 41, 3809 CrossRef CAS; M. S. Han and D. H. Kim, Bull. Korean Chem. Soc., 2004, 25, 1151 CrossRef; R. G. Hanshaw, E. J. O'Neil, M. Foley, R. T. Carpenter and B. D. Smith, J. Mater. Chem., 2005, 15, 2707 RSC; M. K. Coggins, A. M. Parker, A. Mangalum, G. A. Galdamez and R. C. Smith, Eur. J. Org. Chem., 2009, 343 CrossRef.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739 CrossRef CAS.
- K. A. Connors, Binding Constants: The Measurements of Molecular Complex Stability, John Wiley and Sons, 1987 Search PubMed.
- K. M. DiVittorio, J. R. Johnson, E. Johansson, A. J. Reynolds, K. A. Jolliffe and B. D. Smith, Org. Biomol. Chem., 2006, 4, 1966 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and NMR spectra for all new compounds and anion titration data. See DOI: 10.1039/c3cc40937f |
| This journal is © The Royal Society of Chemistry 2013 |
