Enantioselective trapping of phosphoramidate ammonium ylides with iminoesters for synthesis of 2,3-diaminosuccinic acid derivatives†‡
Abstract
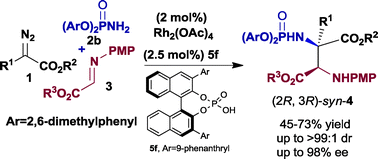
A highly enantioselective trapping of protic phosphoramidate ammonium ylides with α-imino
- This article is part of the themed collection: Emerging Investigators 2013

 Please wait while we load your content...
Please wait while we load your content...