Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Potassium formate as a small molecule switch: controlling oxidation–reduction behaviour in a two-step sequence†
Jorgen S.
Willemsen
,
Jan C. M.
van Hest
and
Floris P. J. T.
Rutjes
*
Institute for Molecules and Materials, Radboud University Nijmegen, Heyendaalseweg 135 6525 AJ NIjmegen, The Netherlands. E-mail: F.Rutjes@science.ru.nl; Fax: +31 243653393; Tel: +31 243653202
First published on 22nd February 2013
Abstract
A one-pot reaction sequence is described in which the addition of one compound allowed switching between simultaneously occurring oxidation and reduction reactions.
In the past decade, multi-step one-pot procedures have attracted increasing interest of the chemical community.1 Combining multiple transformations in one reaction vessel renders sequences more cost-effective as fewer work-up and purification steps are required. Multistep one-pot procedures are therefore considered to contribute to a more sustainable way of producing chemicals. A major challenge in the design of multi-step sequences is the compatibility of the involved substrates, intermediates and reagents or catalysts.
A particular challenge is seen in reactions in which opposing or counteracting reagents or catalysts (acid–base, oxidator–reductor) are used simultaneously.2 Incompatibilities between opposing catalysts have been resolved by immobilization on insoluble polymers3 or encapsulation in sol–gels.4 Such behaviour is advantageously used in for example dynamic kinetic resolutions and related procedures5 in which secondary alcohols are racemised via the corresponding ketones through a redox equilibrium induced by a single catalyst. A remarkable case has been described by Kroutil et al., in which racemisation and reduction were performed stepwise using a single alcohol dehydrogenase (ADH). Switching between non-selective oxidation and enantioselective reduction occurred by removal of the co-substrate acetone and addition of i-propanol.6
In a different strategy, enantioselectivity has been achieved via chemical oxidation by a metal-catalyst, and simultaneous reduction by ADH in the same reaction vessel.7 As shown in this approach, performing multistep reactions in a consecutive manner does not per se require the adaptation of the catalysts or reactants used.
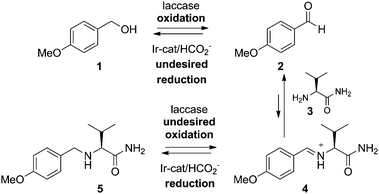
In this communication, we describe a model one-pot reaction system with counteracting catalysts, in which the oxidant acts on the starting compound and the final product, while the reductant acts on the intermediate product leading to both the starting material and the final product. We have shown that by introducing a specific reagent, the reaction can be efficiently steered into one direction by ‘switching-off’ the oxidation and simultaneously ‘switching on’ the reduction, with all catalysts present from the start of the reaction. This is the first example of a reagent-mediated switch between two counteracting catalytic systems, which can now be efficiently performed in one pot in the desired sense.
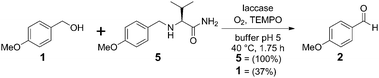
The two-step sequence involves the aqueous enzymatic oxidation of p-methoxybenzyl alcohol (1) to anisaldehyde (2), which in the presence of valinamide (3) is in equilibrium with iminium intermediate 4 (Scheme 1). For the enzymatic oxidation laccase was selected because of its outstanding oxidative performance on benzylic systems and its broad substrate selectivity.8 The iminium ion 4 reacts further in an iridium-catalyzed reductive amination with formate as the hydride source to yield amine 5. However, since the latter product is readily oxidized by laccase, and benzaldehyde is also prone to reduction by the iridium catalyst, this process does not lead to any product formation when carried out in a single vessel. The major challenge therefore was to limit the undesired reverse reactions.
 | ||
| Scheme 1 One-pot oxidation–reduction sequence. | ||
In order to tackle the problem, we initially analysed the partial reactions involved in more detail. Laccase belongs to the class of oxidoreductases and is capable of performing oxidations at the expense of O2 with water as the only byproduct. Because of its stability and non-toxic waste, laccase is also widely applied in industry.9 Laccase readily oxidised anisyl alcohol (1) to aldehyde 2 in oxygenated citric acid buffer at pH 5 in 92% yield (Scheme 2). We selected an activated alcohol10 to keep the reactions short. In addition, 0.3 equivalents of TEMPO were used as an electron carrier, which appeared crucial for the oxidation to proceed.10,11
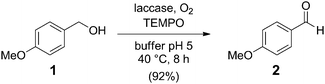 | ||
| Scheme 2 Laccase-mediated oxidation of anisyl alcohol (1). | ||
The reducing agent in our sequence was iridium complex 6 (Scheme 3), which has been successfully applied in aqueous reductive amination reactions.12 The catalyst is based on related iridium complexes which catalyse transfer hydrogenation reactions as has been documented by Fukuzumi et al.13 The reductive amination of 2 with (S)-valinamide (3) to give the stable product 5 proceeded in 92% yield under similar conditions in buffer at pH 5 at 40 °C (Scheme 3). Thus, iridium complex 6 was capable of reducing the intermediate iminium ion 4 in the presence of HCO2K as the hydride source. An excess of anisaldehyde (2) was used, because it was partially reduced to anisyl alcohol (1) in a competing reaction.
 | ||
| Scheme 3 Aqueous reductive amination of anisaldehyde (2). | ||
Next, the compatibility of the oxidation conditions with each of the reactants of the reductive amination was examined (Table 1). The presence of either valinamide (3, entry 2) or iridium complex 6 (entry 3) was tolerated. Contrarily, a 64 mM concentration of HCO2K (1 equiv.) almost completely inhibited the enzymatic activity, while 32 mM HCO2K (0.5 equiv.) resulted in only a small decrease in yield. To investigate whether this inhibition is reversible, we removed HCO2K by filtration over a spin-filter, after which the recovered laccase was tested in the oxidation of alcohol 1. Formation of aldehyde 2 was indeed observed, showing that laccase is only reversibly inactivated by HCO2K.
The same approach was used for the reductive amination (Table 2), which revealed that the reaction was not hampered by TEMPO (entry 2). The presence of laccase (entry 3) did have a tolerable negative influence on the yield. The work of Arends et al. revealed that the rhodium analogue of complex 6 shows a significant decrease in activity in the presence of various enzymes due to complexation of the metal center.14 In our hands the activity loss was limited, because a higher complex/enzyme ratio was used. In the presence of both laccase and TEMPO (entry 4), an initial yield of 8% was observed after 4 h, which gradually decreased to provide only traces of product 5 after 7 h, indicating that the product had reacted with another compound.
Fabbrini and coworkers showed that benzylic amines can be oxidized to iminium ions by the oxidized TEMPO species, resulting in the corresponding aldehydes in aqueous media.11b Likewise, the alkylated valinamide 5 may be rapidly oxidized via the iminium intermediate to aldehyde 2 and valinamide 3 in the presence of laccase and TEMPO. A competition experiment with alcohol 1 and amine 5 confirmed that the amine is more easily oxidised at pH 5.0 to aldehyde 2 than anisyl alcohol (1) (Scheme 4). At the time amine 5 was fully oxidised to aldehyde 2, 63% of anisyl alcohol (1) was still left. Competitive experiments at lower pH also gave a higher rate for the amine oxidation (pH 4.5–3.5) or resulted in laccase inactivation (pH 3.0–2.5).‡
The latter results underline that it is impossible to conduct both transformations simultaneously, and that in a one-pot system the products revert to the starting materials. We then chose to adjust the procedure, prompted by the observed inhibition of laccase by HCO2K (Table 1, entry 4). To test whether laccase could be inactivated and oxidation of amine 5 would be suppressed, the reductive amination was performed in the presence of laccase, TEMPO and 25 equivalents of HCO2K. Indeed, 88% of the desired product was formed, showing that HCO2K could be used as a switch between the oxidation and the reduction.
After establishing the possibility to switch between the two different reactions, we set out to optimize the one-pot oxidation–reduction process (Scheme 5). Reaction in the absence of laccase (Table 3, entry 1) did not lead to any product formation. Without inhibition of laccase – in the presence of small amounts of HCO2K – no desired product was formed either (entry 2). If 100 equivalents of HCO2K were added, laccase was successfully inhibited (entry 3) albeit that only 18% of the desired compound was obtained. The low yield was the result of a second, fast competing reduction, the aldehyde being reduced to alcohol 2. The iminium ion is formed in an unfavourable equilibrium reaction in water, and a fast reduction mainly results in aldehyde reduction. Decreasing the reduction rate should afford more product 5 because more time is available to set the equilibrium. Indeed, when 25 equivalents of HCO2K were used (entry 4), the yields significantly increased. A further enhancement was achieved by using 10 equivalents of HCO2K (entry 5). In this case, a ten-fold lower amount of laccase was used as otherwise the enzyme would not be inhibited efficiently and oxidation of compound 5 would occur. Remarkably, the oxidation of the alcohol was only slightly slower in entry 5 compared to that in entries 2–4. When HCO2K was added in two portions of 2.5 equivalents a yield of 64% was observed. To summarize, by using the appropriate amount of the ‘switching’ compound, the yield was increased from traces to 64%.
 | ||
| Scheme 5 One-pot two-step sequence with opposing catalysts. | ||
| Entry | Laccase (U) | Ir-cat (mol%) | HCO2K (equiv.) | Time for oxidation (h) | Alcohol conversiona,b (equiv.) | Total reaction time (h) | Conversiona (%) |
|---|---|---|---|---|---|---|---|
| a Conversions determined by RP-HPLC. b With respect to valinamide 3. c Isolated yields are given in brackets. Isolated yields were lower due to losses during the extraction procedure. d Two times more concentrated. e Four times more concentrated. | |||||||
| 1 | 0.0 | 5 | 10 | 10 | 0.00 | 10 | 0 |
| 2 | 25.0 | 5 | 0.5 | 24 | 5.00 | 24 | 0 |
| 3 | 25.0 | 5 | 100 | 12.5 | 4.46 | 20 | 18 |
| 4 | 25.0 | 5 | 25 | 12.5 | 4.61 | 20 | 29 |
| 5 | 2.50 | 5 | 10 | 13 | 4.44 | 20 | 32 |
| 6 | 2.50 | 5 | 2 × 2.5 | 16.5 | 4.32 | 65 | 64 |
| 7 | 2.50 | 1 | 10 | 22 | 3.67 | 50 | 80 (40)c |
| 8 | 2.50 | 0.5 | 10 | 23 | 3.78 | 71 | 70 |
| 9 | 2.50 | 0.2 | 10 | 17 | 4.50 | 136 | 61 |
| 10 | 2.50 | 1 | 3 × 2.5 | 23 | 3.78 | 88 | 81 |
| 11d | 2.50 | 1 | 10 | 41 | 3.51 | 65 | 91 (50)c |
| 12e | 2.50 | 1 | 10 | 41 | 2.36 | 41 | 78 (62)c |
We continued the optimisation by varying the amount of catalyst 6 (entries 7–9). The presence of 0.5 or 1 mol% catalyst gave high yields of 70 and 80%, respectively, while 0.2 mol% catalyst led to a disagreeable reaction time of 136 h and a lower yield. In entry 10, optimised amounts of both HCO2K (2.5 equiv.) and iridium complex 6 (1 mol%) were used. However, a comparable yield was obtained in the example in which HCO2K was added in one batch (entry 7).
A last strategy involved using more concentrated reactions, thereby increasing the concentration of iminium ion 4. Indeed, a high yield of 91% (entry 11) was obtained if the reaction was performed twice as concentrated. Contrarily, 78% (entry 12) was found with a four times more concentrated reaction mixture. In the latter case, the enzymatic oxidation appeared to be slow and far from completion, explaining the lower yield. Probably the high concentration of reagents hampered the laccase activity.
Preliminary experiments to apply this switch concept to substituted benzylic alcohols indicated that in each of these cases extensive experimentation was required, which is considered to be beyond the scope of this communication.
A successful two-step reaction sequence with counteracting catalysts in water was performed. An enzymatic oxidation was combined with a reducing metal catalyst in one-pot. First, anisyl alcohol (2) was oxidised to anisaldehyde (3), which reacted in a reductive amination with valinamide (4). The reaction sequence was performed subsequently in which it was crucial to inhibit the laccase with an excess of HCO2K. At the same time, the reductive amination started. The reaction conditions were fine-tuned and led to a maximum conversion of 91% and a maximum isolated yield of 62%. This is the first example in which a compound acts as a switch between two opposing reactions in a one-pot procedure.
Notes and references
- (a) A. Bruggink, R. Schoevaart and T. Kieboom, Org. Process Res. Dev., 2003, 7, 622–640 CrossRef CAS; (b) J. Zhou, Chem.–Asian J., 2010, 5, 422–434 CrossRef CAS PubMed.
- J. H. Schrittwieser, J. Sattler, V. Resch, F. G. Mutti and W. Kroutil, Curr. Opin. Chem. Biol., 2011, 15, 249–256 CrossRef CAS PubMed.
- B. J. Cohen, M. A. Kraus and A. Patchornik, J. Am. Chem. Soc., 1981, 103, 7620–7629 CrossRef CAS.
- (a) F. Gelman, J. Blum and D. Avnir, Angew. Chem., Int. Ed., 2001, 40, 3647–3649 CrossRef CAS; (b) F. Gelman, J. Blum and D. Avnir, New J. Chem., 2003, 27, 205–207 RSC; (c) S. L. Poe, M. Kobašlija and D. T. McQuade, J. Am. Chem. Soc., 2006, 128, 15586–15587 CrossRef CAS PubMed.
- (a) O. Pàmies and J. Bäckvall, Chem. Rev., 2003, 103, 3247–3261 CrossRef PubMed . For some examples, see e.g; (b) A. L. E. Larsson, B. A. Persson and J. Bäckvall, Angew. Chem., Int. Ed., 1997, 36, 1211–1212 CrossRef CAS; (c) D. Cartigny, K. Puntener, T. Ayad, M. Scalone and V. Ratovelomanana-Vidal, Org. Lett., 2010, 12, 3788 CrossRef CAS PubMed; (d) J. Limanto, S. W. Krska, B. T. Dorner, E. Vazquez, N. Yoshikawa and L. Tan, Org. Lett., 2010, 12, 512 CrossRef CAS PubMed.
- C. V. Voss, C. C. Gruber and W. Kroutil, Tetrahedron: Asymmetry, 2007, 18, 276–281 CrossRef CAS.
- F. G. Mutti, A. Orthaber, J. H. Schrittwieser, J. G. de Vries, R. Pietschnig and W. Kroutil, Chem. Commun., 2010, 46, 8046–8048 RSC.
- (a) S. Riva, Trends Biotechnol., 2006, 24, 219–226 CrossRef CAS PubMed; (b) S. Witayakran and A. J. Ragauskas, Adv. Synth. Catal., 2009, 351, 1187–1209 CrossRef CAS; (c) J. M. M. Verkade, L. J. C. van Hemert, P. J. L. M. Quaedflieg, H. E. Schoemaker, M. Schűrmann, F. L. van Delft and F. P. J. T. Rutjes, Adv. Synth. Catal., 2007, 349, 1332–1336 CrossRef CAS.
- (a) S. Rodríguez. Couto and J. L. T. Herrera, Biotechnol. Adv., 2006, 24, 500–513 CrossRef PubMed; (b) A. P. Virk, P. Sharma and N. Capalash, Biotechnol. Processes, 2012, 28, 21–32 CrossRef CAS PubMed.
- M. Fabbrini, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2002, 16, 231–240 CrossRef CAS.
- (a) A. Potthast, T. Rosenau, C. L. Chen and J. S. Gratzl, J. Mol. Catal. A: Chem., 1996, 108, 5–9 CrossRef CAS; (b) M. Fabbrini, C. Galli, P. Gentili and D. Macchitella, Tetrahedron Lett., 2001, 42, 7551–7553 CrossRef CAS.
- J. M. McFarland and M. B. Francis, J. Am. Chem. Soc., 2005, 127, 13490–13491 CrossRef CAS PubMed.
- (a) T. Abura, S. Ogo, Y. Watanabe and S. Fukuzumi, J. Am. Chem. Soc., 2003, 125, 4149–4154 CrossRef CAS PubMed; (b) S. Ogo, K. Uehara, T. Abura and S. Fukuzumi, J. Am. Chem. Soc., 2004, 126, 3020–3021 CrossRef CAS PubMed; (c) S. Ogo, K. Uehara, T. Abura, Y. Watanabe and S. Fukuzumi, J. Am. Chem. Soc., 2004, 126, 16520–16527 CrossRef CAS PubMed.
- M. Poizat, I. W. C. E. Arends and F. Hollmann, J. Mol. Catal. B: Enzym., 2010, 63, 149–156 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc00126a |
| ‡ Details of the competitive oxidation under various pH values can be found in the ESI.† |
| This journal is © The Royal Society of Chemistry 2013 |